Abstract
This research explores the enhancement of single-slope solar still performance using phase change material (PCM), specifically paraffin, incorporating Al2O3 nanoparticles. The application of paraffin, a PCM, improves energy storage density and maintains a consistent temperature during the phase transition. Adding Al2O3 nanoparticles to the PCM improves its thermal properties, increasing production rates. Three scenarios were tested for comparison: (1) a standalone solar still, (2) a solar still with PCM, and (3) a solar still with PCM containing Al2O3 nanoparticles. The productivity yields for these systems were 0.837 kg, 0.924 kg, and 1.145 kg, respectively. The results indicate a significant improvement in the solar still’s performance upon adding PCM and Al2O3 nanoparticles, yielding a 10.38% increase in daily output and a 36.77% increase in daily distillate compared to the standalone solar still. Optimizing the temperature difference between the water and the glass surface through ideal water spraying conditions also bolstered the distillate production rate. The outcomes from this research suggest that solar distillation plants, which provide an efficient source of clean drinking water, can significantly improve performance and productivity by leveraging the benefits of innovative materials such as nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The exponential growth of the population and the escalating energy demand have led to the depletion of fossil fuel reserves, environmental contamination, and increased expenses associated with production and consumption [1]. In light of these challenges, solar energy has emerged as a promising alternative for various applications, including wastewater treatment [2]. However, the intermittent solar irradiation poses a substantial obstacle to the energy management of this technology. Moreover, in areas devoid of contemporary technological advancements, the process of distillation continues to be employed for the purpose of generating drinkable water [3]. This process can benefit from the use of phase change materials (PCMs), which are substances capable of storing and releasing heat during phase changes, such as the transition from solid to liquid. The incorporation of PCMs in distillation systems holds the potential for improving energy efficiency and water purification processes [4]. Bo et al. [5] proposed a new solar-driven PCMs that integrated an interfacial evaporation system to achieve high energy-efficiency desalination. Moreover, this technique proved to be very efficient in utilizing intermittent solar energy. Additionally, PCMs, such as paraffin, salt hydrates, and fatty acids, are frequently employed in various technical applications. In a different study, Liu et al. [6] aimed to improve the performance of PCMs by integrating halloysite nanotubes (HNTs) into organic PCM microcapsules. As a result, the inclusion of HNTs in the PCM microcapsules led to enhanced thermal storage, temperature regulation, flame retardancy, and thermal conductivity of the composite. Another experimental study conducted by Yousef and Hassan et al. [7] focused on solar distillation systems that utilized PCM and wool fibers to increase daytime freshwater output. Through their research, they discovered that PCM significantly improved the exergetic and energetic performance of the distillation system. In the field of desalination processes, nanomaterials have become an increasingly popular choice due to their ability to enhance solar thermal performance [8]. In fact, solar stills, which play a critical role in solar energy systems and offer eco-friendly solutions for hard water or brine treatment, have leveraged the use of nanomaterials to boost their efficiency [9]. Various researchers have employed nanofluids to optimize the performance of solar stills [10]. For instance, Kabeel et al. [11] developed an experimental model of solar still that effectively incorporated an oil heat exchanger and PCMs to substantially increase the production of freshwater. In addition, researchers such as Chaichan et al. [12] have discovered that the addition of nanoparticles to a solar distillation system enhances heat transfer and boosts distillation yield. It has been demonstrated that nanofluids, which consist of nanoparticles dispersed within a base fluid, have the potential to improve the performance and thermophysical properties of heat exchanger fluids [10]. Overall, it is evident that nanomaterials and nanofluids offer optimistic results in solar desalination and the treatment of hard water or brine [10]. Additionally, it has been observed that the incorporation of nanofluids increases the rate of evaporation of nanoparticle/fluid-based solar stills, particularly in regions with limited energy supplies; however, difficulties like long-term stability and life cycle assessment still need to be resolved [13]. The productivity of solar stills depends on efficiency parameters including thermal conductivity, evaporation, and condensation rates, which can be improved by incorporating nanofluids into solar stills [9]. Various design and operational aspects, such as still type, inclination angle, and energy storage, must be taken into account to achieve optimal production [14]. The efficiency and productivity of solar stills can be significantly increased, making them more efficient at addressing the freshwater shortage if correct optimal design considerations are made with the usage of nanofluids [8]. This is corroborated by studies that demonstrate water outputs from 4 kg m−2 to 15 kg m−2 per day may be produced by active solar stills, which function by utilizing solar energy [15]. Additionally, the use of nanofluids in these distillation systems has shown a notable improvement in both thermal conductivity and absorptivity, resulting in a general increase in system efficiency [15]. Overall, the use of nanofluids in solar stills is a promising approach to boosting productivity and efficiency, especially in remote places with a lack of fresh water. Nevertheless, solar stills continue to encounter constraints in terms of their efficiency and the deposition of salt [16]. Numerous studies have been undertaken with the aim of enhancing the efficiency, productivity, and cost-effectiveness of solar stills. A comparative study was conducted in Chennai, India, to assess the energy efficiency of single-slope solar stills utilizing cotton cloth energy storage [17]. The results indicated that the solar still equipped with a 6-mm cotton cloth exhibited the highest energy efficiency, measuring 23.8%. Furthermore, this configuration demonstrated the highest exergy efficiency, reaching 2.6%. Consequently, the implementation of the 6 mm cotton cloth solar still resulted in a notable 24.1% increase in productivity [18]. The investigation determined that a solar still of moderate size, equipped with reflectors and a glass cover for cooling, exhibited superior performance in terms of freshwater productivity and energy efficiency [19]. Pathak et al. investigated a hybrid system for the distillation and production of hot water using a heat pipe-equipped vacuum tube collector and a solar still. The system increased the productivity of distilled water by 152.9% at 40% depth and 162% at 60% depth for variable flow rates. The energy and exergy efficiencies increased by 40–22% and 0.7% to 4.1%, respectively [20]. A comparative study was conducted to evaluate three different configurations of solar stills. The results indicated that the solar stills equipped with a packed layer of glass balls as a thermal storage medium exhibited the highest efficiency, reaching 14.96% [21]. Furthermore, these solar stills demonstrated the potential for integration with parabolic trough collectors, which enhances their viability for deployment in water-scarce regions [21]. Furthermore, an examination was conducted to assess the influence of nanoparticles on the thermal efficiency of single-basin dual-slope solar stills [22]. The findings of the study indicated that the utilization of copper oxide nanoparticles yielded the highest degree of enhancement in the thermal efficiency of solar stills [22]. Subsequent investigations have delved into the utilization of phase change materials (PCMs), wherein passive stills have exhibited enhancements of up to 120%, while active stills have achieved a remarkable improvement of 700% [4]. Moreover, in another investigation researchers suggested that the incorporation of γ-Al2O3 nanoparticles in a solar still can improve the thermal characteristics of saline water and enhance the distillate yield. Specifically, the addition of 0.3 mass% of γ-Al2O3 nanoparticles resulted in a substantial increase in distillate yield [23]. Additionally, a separate study examined the utilization of novel substances, such as phase change material and nanoparticles, in combination with water flow across a glass cover, intending to improve the daily production rate of distilled water in a solar thermal application involving single-slope single-basin solar still [24]. A study conducted in Algeria revealed that the incorporation of CuO nanoparticles into solar stills resulted in enhanced productivity during the winter season [25]. The conventional stills achieved productivity of 3.5 kg m−2 in summer and 2.2 kg m−2 in winter. However, the modified distillery achieved a significantly improved outcome, approximately 1.4 times better, during the winter season [25]. Additionally, this modification led to a notable increase in exergy efficiency, with a 52.5% improvement observed during the winter season [25]. Furthermore, a comparative study was conducted utilizing CuO, Al2O3, Ag, Fe2O3, and SiC-water nanofluids in passive single-slope solar stills. Al2O3-water nanofluid generated 14.22% more thermal energy per day than a simple solar still without nanofluid, followed by CuO (10.82%), Ag (8.11%), Fe2O3 (7.63%), and SiC (7.61%). The outcomes were compared to experimental and theoretical research [26]. Finally, a research study was conducted to examine the influence of the number of baffles on the efficiency of solar stills [27]. The findings of the study indicate that there was a positive correlation between the number of baffles and the increase in water temperature, with a 26.37% rise observed [27]. Additionally, freshwater productivity experienced a boost ranging from 9.7% to 10.925%. These results suggest that increasing the number of baffles can be considered as an effective approach to enhance the efficiency of solar stills [27]. In general, the aforementioned studies provide evidence that the efficiency, productivity, and cost-effectiveness of solar stills can be improved by integrating new materials and inventive designs. This enhancement is particularly valuable in tackling the issues of water scarcity and energy demand [28]. In summary, solar energy has demonstrated its efficacy and cost-effectiveness as a viable alternative to conventional energy sources. Significant advancements have been achieved in enhancing the efficacy of water purification and desalination procedures through the utilization of solar stills and the integration of nanofluids [29]. However, additional research and development efforts are required to address current limitations and enhance the efficiency of solar stills. By effectively tackling these challenges, solar energy has the potential to further contribute to sustainable solutions for the pressing issues of water scarcity and environmental concerns [30]. Despite the extensive body of research on the utilization of PCMs and nanomaterials to enhance solar distillation processes, there is a notable gap in the existing literature regarding the improvement of performance in single-slope solar stills. Previous research has predominantly concentrated on examining either PCMs or nanomaterials with the aim of augmenting the efficiency of solar stills. However, there has been limited research conducted on the utilization of both combined composite materials in a singular inclined solar energy system. The main aim of this study is to enhance the efficiency of water distillation methods, specifically targeting the pressing need for improved access to potable water in remote regions that heavily rely on solar distillation techniques. In order to accomplish this objective, PCM, specifically paraffin, is utilized along with Al2O3 nanoparticles to improve the performance of the single-slope solar still. Furthermore, a comparative analysis is also conducted on standalone solar stills and solar stills integrated with PCM. This distinctive approach effectively assesses both thermal efficiency and distillation productivity, thereby offering valuable insights for potential advancements in this domain.
Experimental setup
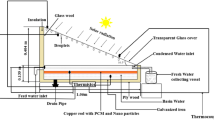
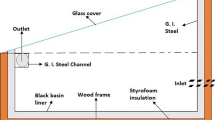
The solar still’s base was constructed using a galvanized iron (GI) painted sheet, whereas the basin was painted black to increase its ability to absorb solar radiation. The apparatus contained water, and as solar radiation permeated the glass enclosure, the water’s temperature gradually rose until it reached a predetermined threshold, prompting evaporation to occur within the distillation apparatus. In order to ensure optimal performance, it is imperative that the basin liner possesses impermeability to leak and exhibits a substantial absorption capacity. Figure 1 illustrates the experimental arrangement, wherein the base was thermally insulated using thermocol material and positioned on a stand with a height of 0.75 m. A layer of black paint was put on the basin’s surface to improve the absorption of solar energy. The basin possessed a surface area measuring 0.185 square meters and was equipped with insulation in the form of a 17-mm thick layer of thermocol material applied to its base. The glass cover was positioned at an inclination of 30 degrees relative to the horizontal plane and securely sealed using silicon sealant and tape. A V-shaped channel comprising three sides was fabricated, accompanied by a 4-cm drainage channel featuring a nozzle with a diameter of 6 mm for water discharge. The liquid measurement cylindrical jar, with an accuracy of ± 0.1 mL, was connected to the outlet through a pipe to collect the distilled water. A comprehensive examination was conducted on the entire setup to ascertain the absence of leaks and optimize heat dissipation. In order to address concerns regarding fragility, a 4-mm glass cover with an average transmissivity of 0.087 was employed as a substitute for high transmissivity glass. The implementation of adequate insulation played a crucial role in ensuring the optimal functioning of the system. Moreover, thermocol was employed on the lower surface to mitigate heat loss, while the side wall was constructed using a GI sheet that had been painted. To further reduce heat loss and enhance the evaporation process, a sealant was employed. Furthermore, to gather the distilled water, a V-shaped channel was affixed to the GI sheet. The installation of a pyranometer set up by the National Institute of Wind Energy was undertaken to quantify wind energy and solar radiations. The data utilized in this experiment were acquired from a website specifically dedicated to global radiation incidents [31]. Using a digital thermosensor manufactured by ApTechDeals, the temperatures of various components of the solar still, including the basin liner, inner glass, and water vapor, were measured. The instrument’s sensitivity was taken into consideration for accurate temperature readings. The thermal sensor exhibited a temperature range spanning from − 50 ℃ to 110 ℃, with an accuracy level of ± 1 °C. Moreover, two sensors were used to measure the properties of the inner and outer glass, as well as four sensors for the sidewalls, one for the basin liner, one for water temperature, and one for water vapor. Finally, the outlet water was gathered utilizing a liquid measurement cylindrical container with a precision of ± 0.1 mL. Table 1 shows the geometric characteristics of the setup.
Materials selection
The basin of the solar still employed commercial-grade paraffin (CAS NO.: 8002–74 − 2) as the PCM. To separate the paraffin from the water, a 0.5-mm-thick aluminum sheet was utilized. Table 2 shows the thermophysical properties of paraffin wax.
Besides this aluminum oxide nanoparticle (Alpha), CAS: 1334–28 − 1 was used as nanomaterials in this experiment.
Experimental procedure
In June 2022, the experimental setup was installed on the rooftop of DTU Delhi, India, to obtain precise and reliable results. The experiment was carried out under clear weather conditions, with the solar still being oriented north–south to measure its entire output accurately. Thermosensors took hourly temperature measurements for the basin liner, cover glass, and water vapor. The estimation of distillate yields on an hourly and daily basis was conducted by evaluating liquid containers. The analysis was conducted between 9 AM and 8 PM. The experiment was conducted in three distinct stages, with each stage focusing on examining different cases. The solar still underwent testing from June 10 to June 13 in the initial instance. The solar still with PCM was tested from June 21 to June 25 in the second case. Finally, the third case study entailed the utilization of a solar still incorporating PCM-Al2O3 from June 26 to June 29. Figure 2 displays the hourly readings that were obtained. Water was sprayed after 5 PM. The aluminum oxide (Al2O3) was uniformly distributed onto the PCM with a mass percentage of 2% aluminum oxide. The experimental procedure initially subjected the wax to heat until it reached its melting point. Subsequently, an approximate quantity of 5 g of aluminum particles was evenly dispersed within the container, which was then subjected to agitation for 60 min. Following the cessation of the heat supply, an approximate quantity of 5 g of aluminum oxide was dispersed onto the molten wax, resulting in its solidification. Subsequently, an additional quantity of 10 g was evenly distributed onto the upper surface of the solidified wax. The required amount of nanoparticles can be calculated below the equation:
where Ø represents the mass fraction of the nanoparticle, \({m}_{\text{PCM}}\) denotes the mass of the PCM, and mn− Al2O3 indicates the mass of the nanoparticle that has been introduced to the PCM. The consistent and enduring diffusion of nanoparticles into paraffin wax poses significant challenges in preserving the thermophysical characteristics of the mixture. Consequently, even a small dispersion of nanoparticles can significantly impact the properties of the mixture. This approach entails preserving consistency within solutions containing wax and nanoparticles.
The hourly efficiency calculated by the given formula
where, A is the area of solar still (m2), I is the intensity of solar radiation (W m−2), P is the productivity (m−3), and hfs is the latent heat of the distiller water.
Result and discussion
The experimental observation was carried out in Delhi (Latitude: 28.7°N, 77.10°E) from June 10, 2022, to June 13, 2022, without PCM. In the second case, an experiment was conducted with a layer of PCM on the basin bed from June 21 to June 25, 2022. In the third case, another experiment was conducted using PCM–Al2O3. An appropriate instrument was used to measure all the temperatures. Figure 2 displays the global solar radiation variation for the solar still alone. The values for June 10 and June 11 range from 700 to 900 W m−2 during the 9-AM to 2-PM interval. Figure 3 shows the global solar radiation variation for the solar still with PCM. The values for June 21 and June 24 range from 550 to 750 W m−2 in the 9-AM to 2-PM interval. Figure 4 provides an overview of the global solar radiation variation for the solar still with PCM–Al2O3. The values for June 27 and June 29 range from 650 to 900 W m−2 within the 9-AM to 2-PM interval.
Figure 5 displays the estimated solar irradiation observed for a solar still without PCM on June 11, a solar still with PCM on June 21, and a solar still with PCM–Al2O3 on June 27. The solar radiation during this period was determined to be approximately the same. Figure 5 shows the radiation variation in different scenarios: which is still alone, one with PCM, and one with PCM–Al2O3. The figure demonstrates that the solar radiation is nearly identical for the specific date used in this analysis. This radiation data was utilized to compare various parameters of the solar still.
Figure 6 displays the distilled output data for three different configurations: solar still alone, solar still with PCM, and solar still-PCM–Al2O3. The findings suggest that productivity is enhanced during off-peak periods, specifically in the context of solar still- PCM–Al2O3. The results demonstrate an overall increase in productivity during both on and off-peak times. Figure 7 illustrates the fluctuations in distilled water in response to solar radiation across various scenarios, namely the use of a standalone still, a still incorporating PCM, and a still incorporating PCM–Al2O3. The graph indicates that the distilled water output of the still with PCM–Al2O3 is greater than that of both the still alone and the still-PCM. Figure 8 illustrates the fluctuations in basin water temperature for three different scenarios: solar still alone, solar still with PCM, and solar still with PCM–Al2O3. The graph illustrates that the rate of increase in basin water temperature is significantly higher in solar stills equipped with PCM and solar stills incorporating both PCM and Al2O3, compared to solar stills operating without these enhancements.
The comparison between the hourly solar still performance with the incorporation of PCM and PCM–Al2O3, and the performance of the solar still alone, is shown in Fig. 9. The solar still’s yield was measured at 837 mL m−2 day−1, 924 mL m−2 day−1, and 1145 mL m−2 day−1 for the solar still alone, solar still with PCM, and solar still with PCM and Al2O3, respectively. The increase in yield was approximately 10.38% and 36.77%, respectively. The solar still yielded the highest quantity of distilled water at noon due to the prevailing hot weather conditions. The nocturnal condensed product of the distillation process resulted in an augmentation of solar energy production and an enhancement of the overall distillation output. At 5 PM, water was sprayed onto the glass in order to sustain the temperature differential and optimize the distillation process. Figure 9 illustrates the fluctuations in the external temperature of the glass for three scenarios: the solar still alone, the solar still with PCM, and the solar still with PCM and Al2O3. It is evident from the graph that the outside glass temperature of the solar still with Al2O3 is higher compared to both the solar still alone and the solar still with PCM.
Figure 10 shows the variation of the inside temperature of the glass concerning the hourly time for different cases: solar still alone, still-PCM, and still- PCM–Al2O3. The results indicate that the temperature inside the glass for the solar still-PCM and still- PCM–Al2O3 cases was slightly higher than the other two cases. Figure 11 displays the hourly measurement of water vapor for various cases. The graph indicates that the variation of water vapor ranges from approximately 30 ℃ to 36 ℃ across different cases.
Figure 12 depicts the hourly measurements of the temperature variation on the bottom surface of the still basin for three different configurations: solar still alone, solar still with PCM, and solar still with PCM and Al2O3. The graph illustrates the range of temperature variations observed on the lower surface, which spans from 45 ℃ to 68 ℃. It was noted after careful observation that the basin’s temperature experienced a slight decrease. This can be attributed to the temperature reaching the melting point of the PCM, which is within the range of 58 ℃ to 60 ℃. Between the hours of 12:00 PM and 3:00 PM, the behavior of paraffin wax was characterized by undercharging conditions. Upon completion of the charging process, the glass is covered using a protective cover, and the PCM discharge is underway. Subsequently, the stored energy is transferred to the activated solar still during nighttime. A marginal decrease in yield was observed during the day when comparing the use of still and non-still PCM that is stored below the basin.
Figure 13 illustrates the relationship between the water temperature in the basin and the temperature of the glass placed inside. This suggests that it is important to maintain a distinction to achieve high water distillation productivity for three different systems: solar still alone, solar still with PCM and solar still with PCM and Al2O3. The process of distillation conducted by the simple apparatus yields a greater volume of purified water during the time period spanning from 8 to 11 AM. However, the production of other distillates is expected to increase after this hour. This increase in production results in a portion of the heat being transferred to the wax, causing the temperature of the wax to rise gradually. Consequently, the temperature of the water decreases due to the high capacity of the wax to absorb heat. According to the findings, the temperature of the bottom surface increased to a greater extent compared to the temperatures of the PCM and nano-PCM during the charging phase. This observation aligns with the well-established understanding that metals have a higher heat absorption rate than other materials. During the period of discharge, there was a rapid decrease in temperature observed on the bottom surface in comparison to the paraffin wax. Although the majority of the temperature data obtained from the plates and subsequently transferred to the wax is typically stored as sensible heat and latent heat. Incorporating a solar still involves the utilization of Al2O3–PCM to achieve a slightly higher temperature than the temperature observed when using PCM alone. The incorporation of Al2O3 in the PCM resulted in an enhancement of thermal conductance. This improvement allows the PCM to efficiently accumulate more heat energy within a shorter duration, making it suitable for integration into solar stills. Therefore, using Al2O3–PCM in solar stills results in a higher distillation efficiency compared to solar stills without PCM. Additionally, PCM’s presence impacts the temperature of the water inside the solar still.
Comparison with the previous study
The findings of this study build upon the work of Ramzy et al. [32], who investigated the performance of solar stills using various absorbing materials, including steel wool pads. Ramzy et al. reported a thermal efficiency of 32.74% and a yield of 4.384 l m−2 using steel wool pads as the absorbing material. In contrast, the current research improved solar still performance by integrating PCM with Al2O3 nanoparticles. This method significantly increased thermal efficiency to 36.77% and a maximum water yield of 1.245 kg using the PCM–Al2O3 composite. Therefore, the findings of this study suggest that incorporating nanoparticles into PCM can potentially enhance the efficiency of solar distillation systems.
Conclusions
This study investigates solar distillation systems, focusing on the integration of PCM and PCM–Al2O3. The results show that adding PCM and Al2O3 nanoparticles significantly improved solar still performance. Adding Al2O3 significantly enhanced the thermal performance of paraffin wax, boosting distilled water yield by 10.38%. The daily distillate yield increased by 36.77% when Al2O3 nanoparticles were included in the PCM design. The PCM–Al2O3 composite also showed lower charging and discharging periods, indicating improved thermal storage. Maintaining an ideal water spray rate on the glass surface also increased the output rate, allowing for a more favorable temperature difference between the water and glass surface, boosting the condensation rate. Future studies will explore potential yield increases by swapping Al2O3 for other materials like titanium oxide and copper oxide. The urgent requirement to enhance the efficiency of solar distillation facilities, which hold the potential to provide uncontaminated potable water, underscores the significance of ongoing investigations into novel materials for solar-driven water purification systems.
Data availability
No data associated with this study.
Abbreviations
- PCM :
-
Phase change material
- I :
-
Intensity of solar radiation/W m−2
- P :
-
Productivity/m−3
- A :
-
Area/m2
- n :
-
Nanoparticle
- η :
-
Efficiency
- ϕ :
-
Mass fraction of the nanoparticle
References
Akram N, Sadri R, Kazi SN, Nashrul M, Zubir M, Ridha R, Ahmed W, Soudagar MEM, Arzpeyma M. A comprehensive review on nanofluid operated solar flat plate collectors. J Therm Anal Calorim. 2020;139:1309–43.
Pathak AK, Tyagi VV, Anand S, Pandey AK, Kothari R. Advancement in solar still integration with phase change materials-based TES systems and nanofluid for water and wastewater treatment applications. J Therm Anal Calorim. 2022;147:9181–227.
Khan Y, Raman R, Rashidi MM, Caliskan H, Chauhan MK, Chauhan AK. Thermodynamic analysis and experimental investigation of the water-spray cooling of photovoltaic solar panels. J Therm Anal Calorim. 2023;148:5591–602.
Omara AAM, Abuelnuor AAA, Mohammed HA, Khiadani M. Phase change materials (PCMs) for improving solar still productivity: a review. J Therm Anal Calorim. 2020;139:1585–617.
Gong B, Yang H, Wu S, Tian Y, Yan J, Cen K, Bo Z, Ostrikov KK. Phase change material enhanced sustained and energy-efficient solar-thermal water desalination. Appl Energy. 2021;301:117463.
Liu Y, Kang M, Lin W, Liang C, Qu W, Wang Y, Guan Y, Cheng J. Enhanced thermal performance of phase change material microcapsules using halloysite nanotubes. Appl Therm Eng. 2023;231:120965.
Khan Y, Raman RR, Rashidi MM, Said Z, Caliskan H, Hoang AT. Thermodynamic and exergoenvironmental assessments of solar-assisted combined power cycle using eco-friendly fluids. J Therm Anal Calorim. 2024;149:1125–39.
Vaithilingam S, Gopal ST, Srinivasan SK, Manokar AM, Sathyamurthy R, Esakkimuthu GS, Kumar R, Sharifpur M. An extensive review on thermodynamic aspect based solar desalination techniques. J Therm Anal Calorim. 2021;145:1103–19.
Seyednezhad M, Sheikholeslami M, Ali JA, Shafee A, Nguyen TK. Nanoparticles for water desalination in solar heat exchanger. J Therm Anal Calorim. 2020;139:1619–36.
Okonkwo EC, Wole-Osho I, Almanassra IW, Abdullatif YM, Al-Ansari T. An updated review of nanofluids in various heat transfer devices. J Therm Anal Calorim. 2021;145:2817–72.
Kabeel AE, Abdelgaied M. Observational study of modified solar still coupled with oil serpentine loop from cylindrical parabolic concentrator and phase changing material under basin. Sol Energy. 2017;144:71–8.
Chaichan MT, Kazem HA. Single slope solar distillator productivity improvement using phase change material and Al2O3 nanoparticle. Sol Energy. 2018;164:370–81.
Rashidi S, Karimi N, Mahian O. A concise review on the role of nanoparticles upon the productivity of solar desalination systems. J Therm Anal Calorim. 2019;135:1351145–59.
Jathar LD, Ganesan S, Shahapurkar K, Soudagar MEM, Mujtaba MA, Anqi AE, Farooq M, Khidmatgar A, Goodarzi M, Safaei MR. Effect of various factors and diverse approaches to enhance the performance of solar stills: a comprehensive review. J Therm Anal Calorim. 2022;147:4491–522.
Dharamveer, Samsher, Singh DB, Singh A, Kumar N. (2019) Solar Distiller Unit Loaded with Nanofluid—A Short Review. Lecture Notes in Mechanical Engineering. https://doi.org/10.1007/978-981-13-6577-5_24
Elgendi MA. Review of the Applications of Nanomaterials to Augment Solar Still Productivity. Water Resources Management and Sustainability: Water Science and Technology Library; 2023. https://doi.org/10.1007/978-3-031-24506-0_30.
Sathish D, Jegadheeswaran S. Evolution and novel accomplishments of solar pond, desalination and pond coupled to desalination systems: a review. J Therm Anal Calorim. 2021;146:1923–69.
Sakthivel TG, Arjunan TV. Thermodynamic performance comparison of single slope solar stills with and without cotton cloth energy storage medium. J Therm Anal Calorim. 2021;137:351–60.
Sharshir SW, Salman M, El-Behery SM, Halim MA, Abdelaziz GB. Enhancement of solar still performance via wet wick, different aspect ratios, cover cooling, and reflectors. Int J Energy Environ Eng. 2021;12:517–30.
Pathak AK, Chopra K, Tyagi VV, Anand S, Kothari A, Sari A, Pandey AK. Solar heat pipe ETC integrated with solar still system for water treatment and hot water production: novel hybrid experimental approach. J Therm Anal Calorim. 2023;148:8969–89.
Madiouli J, Saleel CA, Lashin A, Badruddin IA, Kessentini A. An experimental analysis of single slope solar still integrated with parabolic trough collector and packed layer of glass balls. J Therm Anal Calorim. 2021;146:2655–65.
Modi KV, Jani HK, Gamit ID. Impact of orientation and water depth on productivity of single-basin dual-slope solar still with Al2O3 and CuO nanoparticles. J Therm Anal Calorim. 2021;143:899–913.
Faridani HZ, Ameri A. Performance enhancement of a basin solar still using γ-Al2O3 nanoparticles and a mixer: an experimental approach. J Therm Anal Calorim. 2022;147:1919–31.
Suresh C, Shanmugan S. Effect of water flow in a solar still using novel materials. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08449-5.
Attia MEH, Karthick A, Manokar AM, Driss Z, Kabeel AE, Sathyamurthy R, Sharifpur M. Sustainable potable water production from conventional solar still during the winter season at algerian dry areas: energy and exergy analysis. J Therm Anal Calorim. 2021;145:1215–25.
Dhindsa GS, Kumar V, Mittal MK, Sokhal GS, Khalilpoor N, Sharifpur M, Issakhov A, Tonk R. Performance comparison of single-slope solar still loaded with various nanofluids. Energy Sci Eng. 2022;10:4318–31.
Al-Adel Z, Bouabidi A, Chrigui M. 3D CFD simulation and experimental validation of the baffle number effect on the solar still performance. J Therm Anal Calorim. 2023;148:2171–88.
Mohammed AH, Shmroukh AN, Ghazaly NM, Kabeel AE. Active solar still with solar concentrating systems. Review J Therm Anal Calorim. 2023;148:8777–92.
Kumar TRS, Jegadheeswaran S, Chandramohan P. Performance investigation on fin type solar still with paraffin wax as energy storage media. J Therm Anal Calorim. 2019;136:101–12.
Shoeibi S, Rahbar N, Esfahlani A, Kargarsharifabad H. Energy matrices, economic and environmental analysis of thermoelectric solar desalination using cooling fan. J Therm Anal Calorim. 2022;147:9645–60.
https://nsrdb.nrel.gov/ accessed on 12/07/2022.
Ramzy K, Abdelgaleel M, Kabeel AE, Mosalam H. Performance of a single slope solar still using different porousabsorbing materials: an experimental approach. Environ Sci Pollut Res. 2023;30:72398–414.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, Y., Said, Z., Raman, R. et al. Improving single-slope passive solar still efficiency through integration of phase change materials and Al2O3 nanoparticles. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13558-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13558-x