Abstract
Polylactide (PLA) was plasticized with a new biodegradable macromolecular plasticizer-poly(diethylene glycol adipate) (PDEGA). The crystallization behavior, miscibility, rheological behavior, mechanical properties and phase behavior of PLA/PDEGA blends were investigated. The PDEGA lowered the glass transition temperature and the cold crystallization temperature. With an increase of PDEGA content, the break strain and impact strength increased significantly. The high break strain of 480 % and the high impact strength of 30 kJ/m2 were obtained for 70/30 PLA/PDEGA blend. PDEGA was uniformly dispersed in the PLA matrix. The results indicated that PDEGA had a good plasticizing effect on PLA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polylactide (PLA) is produced from renewable resource and has become a useful material, especially in packaging applications due to its good transparency, high strength, and moderate barrier properties. PLA resins have mostly been used for biomedical applications such as drug delivery systems and packaging applications such as films and food containers [1]. Unfortunately, the application of PLA as a substitution of commodity plastics is restricted by its brittle behavior under impact loads [2]. Likewise, the processing temperature window of PLA is narrow. Therefore, much work has been done to modify the brittleness of PLA. Blending PLA with other polymers such as poly(propylene-glycol) [3, 4], poly(ethylene glycol) [5–8] or poly(ethylene oxide) [9, 10] is an effective approach to improve the flexibility of PLA.
There are many demands put on plasticizer, the miscibility is very important. The plasticizer should be miscible with PLA, to create a homogeneous blend. Furthermore, the plasticizer should not be prone to migration, because the migration will lead to pollution of the materials in contact with the plasticized PLA and the embrittlement of plasticized blends [11]. The present study focused on the use of biodegradable macromolecular plasticizer as a modifier for PLA and its effects on the dynamic mechanical and thermal properties of the polymer.
Poly(diethylene glycol adipate) (PDEGA) is a viscous liquid, and it has a low T g , nontoxic and solvent resistance. Using nontoxic biodegradable macromolecular plasticizer is helpful to our environment [12]. These excellent performances make PDEGA attractive potential plasticizer for PLA, up to date, PDEGA plasticized PLA has been reported by Okamoto et al. [13]. The present studies focused on the use of biodegradable macromolecular plasticizer as a modifier for PLA. The thermal, rheological, and mechanical properties of PLA/PDEGA blends were measured.
Experimental
Materials
PLA (2002D) used in this study was supplied by Nature Works, with 4 % meso content (96 % L) and weight-average molecular weight 121,400 g/mol. PDEGA was synthesized by our laboratory, its had weight-average molecular weight 19,000 g/mol, the polydispersity index of 2.5, its viscous value was 12.4 Pa.s.
PLA and PDEGA were melt-blended using a Haake Rheomix 600 (Karlsruhe, Germany) in ratios of 100/0, 95/5, 90/10, 85/15, 80/20, 75/25, and 70/30 w/w. The processing condition was the rotating speed of 60 rpm at 180 °C for 5 min. After that, all of samples were compression-molded into sheets with thicknesses of 1.0 and 4.0 mm at 185 °C, a hold pressure of 10 MPa and a hold time of 5 min.
Characterization
Thermal analysis
DSC was performed using a TA Instruments DSC (Q20) under nitrogen atmosphere. The samples (about 7.0 mg) were heated first from −70 to 185 °C at 10 °C/min, and held at 185 °C for 3 min, then cooled at the same rate and heated again. The degree of crystallinity of the samples was evaluated from the heat evolve during crystallization by the following Eq. (1):
where \(\chi_{\text{c}}\)was the degree of crystallinity of the samples, \(\Updelta H_{\text{f}}\)was the heat of fusion of the blend, \(\Updelta H_{{{\text{f}} }}^{0}\)was the heat of fusion for 100 % crystalline PLA (93 J/g) [14], and \(w_{\text{PLA}}\)was the weight fraction of PLA in the blend.
DMA was carried out on a NETZSCH DMA 242C (Selb, Germany). All samples were cut from the tensile bar specimens (20 × 4 × 1 mm). The experiment was carried out in tension mode at a constant heating rate of 3 °C/min and a frequency of 3.33 Hz, from −90 to 125 °C.
Rheological behaviors testing
Rheological texts were carried out on a Physica MCR 2000 rheometer (AR 2000ex USA). Frequency sweep for the PLA/PDEGA samples was carried out under nitrogen using 25-mm plate–plate geometry. The gap distance between the parallel plates was 0.8 mm for all tests. The sheet samples about 1.0 mm in thickness were melted at 170 °C. The frequency sweep experiments were also carried out within the frequency range of 0.1–100 rad/s at a strain of 1.25 %.
Mechanical properties
Notched Izod impact tests were performed at 23 ± 2 °C according to GB 1843–2008 on an impact testing machine (CEAST, Chengde, China). The samples (80 × 10 × 4 mm) were obtained from compression-molded specimens. The notch was milled in having a depth of 2 mm, an angle of 45° and a notch radius of 0.25 mm. The uniaxial tensile tests were carried out at 23 ± 2 °C on an Instron 1121 testing machine (Canton MA). Specimens (20 × 4 × 1 mm) were cut from the previously compression-molded specimens. The measurements were carried at a cross-head speed of 50 mm/min according to ASTM D638–2008. At least five runs for each sample were measured, and the results were averaged.
Morphological characterization
The cryo-fractured surfaces of PLA/PDEGA blends selected alcohol removal of PDEGA component. The etching time was 30 min. Cryo-fractured surfaces and Izod–fracture surfaces of PLA/PDEGA blends were characterized by SEM (model Japan JXA–840 ESEMFE). All samples were coated with a thin layer of gold of about 10 nm and an accelerating voltage of 10 kV was used to produce the SEM photographs.
Results and discussion
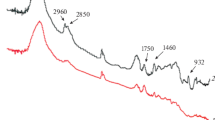
PDEGA was synthesized via polyesterification reaction using diethylene glycol and adipic acid as raw materials (Scheme 1). The polyesterification reacted for 4 h at 140 °C, polycondensation reacted for 2 h at 200 °C. The FTIR spectra of the pure PDEGA showed in Fig. 1. The peak at 1,000 cm-1 corresponded to the C–H stretching vibration absorption, the peak at 1,750 cm-1 corresponded to the ester carboxylic strong stretching vibration absorption, and peak at 1,170 cm-1 corresponded to the C–O of ester stretching vibration absorption. The three absorption peaks strongly evidenced ester exist in the PDEGA.
Thermal properties
The thermal behavior of PLA/PDEGA blends was investigated. Figure 2 shows the second heating curves and the specific thermodynamic parameters are listed in Table 1. The glass transition temperature gradually decreased to lower temperature with the increasing PDEGA content in the PLA/PDEGA blends. The result could be attributed to the increasing mobility of PLA chains, generated by PDEGA. It could be seen from the DSC thermograms that the cold crystallization temperature (T cc) of PLA decreased by the addition of PDEGA, which suggested that PDEGA enhanced the ability of cold crystallization of PLA. It was observed that the cold crystallization temperature of PLA decreased in parallel with the shift in T g. Such decrease in T g and an enhanced cold crystallization process were commonly observed for plasticized PLA systems due to the increased segmental mobility of the PLA chains by plasticization [15–17].
With the addition of PDEGA, there appeared two melting peaks in the thermograms. The two melting peaks of PLA could be attributed to the formation of crystallites with different sizes and perfection, which was improved by lamellar rearrangement during crystallization of PLA [2, 4, 15]. It could be noted that the melting temperature of the lower peak and its size gradually decreased with the increased PDEGA content in the blend. The higher peak gradually increased, and the formation of crystallites more perfected [18].
Fox equation was used to further discuss the PLA/PDEGA system. The Fox equation [2] was as follow:
where W i is weight fraction of pure component i, T g is the blend and T gi is the glass transition temperature of pure component i. Figure 3 shows that when the content of PDEGA was lower 10 wt %, the variation of T g fitted the Fox equation, and the result indicated that the PDEGA was miscible with PLA at low content. However, when the content of PDEGA exceeded 15 wt%, the experimental results had the similar decreased trend and gradually deviated from theoretical value.
DMA experiments were carried out to further test the miscibility of PLA/PDEGA blends. Variations of dynamic storage modulus (E’), loss factor (tanδ), and loss modulus (E’’) were shown in Fig. 4. From Fig. 4a, it could be seen that with the increasing PDEGA content, there was a large drop of the storage modulus around 20–60 °C corresponding to the glass transition region. The results were due to the presence of micro domains of PDEGA, thus allowing for easier chain folding and crystallization of PLA. A similar result was also found by other researchers [19–21]. Then E’ started to raise around 70 °C. Such a rise in E’ was due to cold crystallization.
Figure 4b shows the loss modulus of PLA/PDEGA blends. The full width-at-half-maximum (FWHM) of loss modulus was calculated. The parameters of width are listed in Table 2. The transitional width of PLA increased from 4.3 to 11.1 °C in DSC and the FWHM of PLA increased from 6.2 to 32.9 °C in DMA. The transitional width of PLA decreased from 6.1 to 2.8 °C and the FWHM of PDEGA decreased from 8.4 to 6.4 °C. Likewise, the Hildebrand solubility parameter was used to further discuss the PLA/PDEGA system. The Hildebrand solubility parameter equation [22, 23] was as follow:
where δ is the solubility parameter, E coh is the cohesive energy density and V is the per unit molar volume. From Eq. (3), the solubility parameter of PDEGA was 16.3. The solubility parameter of PLA was 17.6, which had been reported by Agrawal et al. [22]. The results indicated that PLA was partially miscible with PDEGA.
Figure 4c, d presents the glass transition of PLA and PDEGA blends. The tanδ curves revealed two glass transitions, with the higher T g corresponding to PLA component, and the lower one to PDEGA component. When the blend composition was varied, the two T gs of PLA and PDEGA components in the blends showed shifting to lower temperature. The results of DMA suggested that PLA was partially miscible with PDEGA, it was consistent with the results of DSC. The T g data obtained from DMA analysis were different from DSC characterization. This was the result from different test methods used.
Rheological properties
Rheometry has been proved to be a powerful tool for investigating the internal microscopic structure of polymer blend systems. Figure 5a shows the dependence of the dynamic storage modulus (G’) on the frequency (ω) for the PLA/PDEGA blends. At the low frequencies, the G’ of all blends presented lower values dependency in contrast to that of pure PLA. At high frequencies, all samples showed a higher G’. As shown in Fig. 5b, the processed PLA exhibited a Newtonian behavior, with a zero-shear rate viscosity value (around 4,000 Pa.s). With an increase of PDEGA content, the complex viscosity (\(\left| {\eta^{*} } \right|\)) decreased significantly. For example, at 10 rad/s, the complex viscosity of the 70/30 PLA/PDEGA blend was reduced by about an order of magnitude compared to that of pure PLA. The decreased complex viscosity was attributed to droplet size and concentration of PDEGA increase, the SEM micrographs provided this information. Likewise, the decreased complex viscosity of the blends indicated that the process-ability of PLA was improved observably. The addition of plasticizer decreased the intermolecular force, and increased the mobility of the polymeric chains [18, 24, 25].
Morphological and mechanical properties
For the PLA/PDEGA blends, dispersions were studied by SEM in Fig. 6. The black holes could be formed by the removal of PDEGA in cryo-fractured surfaces of the blends. From Fig. 6a, b, the surfaces were smooth. As shown in Fig. 6c–f, the PDEGA was uniformly dispersed in the PLA matrix, and a further increase in the PDEGA content led to average particle size increase. Empty micro-voids were observed in the blends indicating a formation of PDEGA rich phase in PLA matrix by phase separation. Similar phase-separated morphology was also observed in PLA/ESO (epoxidized soybean oil) and PLA/PPG [poly(propylene-glycol)] blends in which low molecular weight was accumulated during separation and form micro-voids in the PLA matrix [4, 16]. The size and the distribution of the particles were exhibited in Fig. 7. From Fig. 7a, PDEGA was uniformly dispersed in the PLA matrix, the size of the particles was about 0.3 μm for 85/15 PLA/PDEGA blend. Figure 7b–d shows that the size of the particles increased with the increasing PDEGA content. The size of the particles was about 2.8 μm for 70/30 PLA/PDEGA blend.
The stress–strain curves of pure PLA and PLA/PDEGA blends were shown in Fig. 8. The mechanical performance parameters are listed in Table 3. The slope of Young’s modulus curve is the common method of determining the stiffness [26–28]. The addition of PDEGA significantly decreased the modulus value from 1,776 to 587 MPa. The yield stress decreased from 58.0 MPa for pure PLA to 26.4 MPa for the 70/30 PLA/PDEGA blend; however, the yield strain increased from 4.1 to 6.6 %. The minimal break stress was 22.8 MPa for 85/15 PLA/PDEGA blend. Pure PLA, 95/5 and 90/10 PLA/PDEGA blends were fractured at low strain, the 85/15, 80/20, 75/25, and 70/30 PLA/PDEGA blends exhibited a ductile behavior with yielding; the ductile behavior was supported by SEM result. These results were believed that the microdomains of PDEGA, decreased the degree of entanglement in PLA, softened the samples, and enhanced the plastic deformation. In addition, the plastic deformation was the major energy-dissipation process and led to a toughened, polymer blend [10].
As shown in Fig. 9, the impact strength increased from the 2.9 kJ/m2 for pure PLA to the 30 kJ/m2 for 70/30 PLA/PDEGA blend. The brittle-ductile transition of the blends was obtained when the PDEGA contents varied from 15 to 20 wt%. When the PDEGA exceed 15 wt%, the impact strength showed a substantial increase, this marked the transition from homogeneous system to a phase-separated system. PLA/PDEGA blends took place in plastic deformation and shear yielding. In addition, PDEGA was a viscous liquid at room temperature, the impact behavior of this system was also thought of as a rubber-toughened thermoplastic, isolated PDEGA domains acting as isolated rubbery domains.
As shown in Fig. 10, the impact fracture surfaces of PLA/PDEGA blends were quite different. Figure 10a shows the characteristic of brittle fracture for 85/15 PLA/PDEGA blend. When PDEGA content was above 15 wt%, the fracture surface of the blends demonstrated that numerous cavities and layers were drawn out of the polymer, as shown in Fig. 10b–d. The micrographs analysis of the impact fracture surface confirmed the brittle-ductile transition in PLA/PDEGA blend. PLA was toughened by PDEGA. The SEM observation was well consistent with the results of mechanical properties. The results indicated that PDEGA had a good plasticizing effect on PLA.
Conclusions
In this study, PLA could be plasticized and toughened by incorporating PDEGA. The results from DSC and DMA showed that PDEGA was partially miscible with PLA. The PLA/PDEGA blends exhibited that there was a significantly dependence of fluidity on composition. The complex viscosity of the PLA/PDEGA blends decreased with the increasing of PDEGA content. The break strain increased and the Young’s modulus decreased significantly which indicated the plasticizing effect of the PDEGA on PLA. For example, comparing to the break strain of pure PLA, the 70/30 PLA/PDEGA blend increased by about 106 times. The impact strength was also improved from 2.9 kJ/m2 for pure PLA to 30 kJ/m2 for the 70/30 PLA/PDEGA blend. SEM results of PLA/PDEGA blends showed that PDEGA was uniformly dispersed in the PLA matrix. Therefore, PDEGA could be used effectively as a modifier of PLA.
References
Martin O, Averous L (2001) Poly(lactic acid): plasticization and properties of biodegradable multiphase systems. Polymer 42:6209–6219
Nijenhuis AJ, Colstee E, Grijpma DW, Pennings AJ (1996) High molecular weight poly(l-lactide) and poly(ethylene oxide) blends: thermal characterization and physical properties. Polymer 37:5849–5857
Kulinski Z, Piorkowska E, Gadzinowska K, Stasiak M (2006) Plasticization of poly(l-lactide) with poly(propylene glycol). Biomacromolecules 7:2128–2135
Piorkowska E, Kulinski Z, Galeski A, Masirek R (2006) Plasticization of semicrystalline poly(l-lactide) with poly(propylene glycol). Polymer 47:7178–7188
Jacobsen S, Fritz HG (1999) Plasticizing polylactide: the effect of different plasticizers on the mechanical properties. Polym Eng Sci 39:1303–1310
Baiardo M, Frisoni G, Scandola M, Rimelen M, Lips D, Ruffieux K, Wintermantel E (2003) Thermal and mechanical properties of plasticized poly(l-lactic acid). J Appl Polym Sci 90:1731–1738
Sheth M, Kumar RA, Dave V (1997) Biodegradable polymer blends of poly(lactic acid) and poly(ethylene glycol). J Appl Polym Sci 66:1495–1505
Kulinski Z, Piorkowska E (2005) Crystallization, structure and properties of plasticized poly(l-lactide). Polymer 46:10290–10300
Jia ZY, Han CY, Dong LS (2009) Thermal-mechanical properties and rheological behaviors of polylactide/poly(ethylene oxide) blends. Acta Polymerica Sinica 20:967–972
Xu YQ, Qu JP (2009) Mechanical and rheological properties of epoxidized soybean oil plasticized poly(lactic acid). J Appl Polym Sci 112:3185–3191
Ren ZJ, Dong LS, Yang YM (2006) Dynamic mechanical and thermal properties of plasticized poly(lactic acid). J Appl Polym Sci 101:1583–1590
Wu XF, Zhao JB, Yang WT (2001) Synthesis method of biodegradable polydiethylene glycol adipate with high molecular weight. J Beijing Univ Chem Technol 28:23–26
Okamoto K, Ichikawa T, Yokohara T, Yamaguchi M (2009) Miscibility, mechanical and thermal properties of poly(lactic acid)/polyester-diol blends. Eur Polym J 45:2304–2312
Fischer EW, Sterzed HJ, Wegner G (1973) Investigation of structure of solution grown crystals of lactide copolymers by means of chemical-reactions. Kolloid Zeitschrift. Polymer 251:980–990
Ge HH, Yang F, Hao YP, Wu GF, Zhang HL, Dong LS (2013) Thermal, mechanical, and rheological properties of plasticized poly(l-lactic acid). J Appl Polym Sci 127:2832–2839
Ali F, Chang YW, Kang SC, Yoon JY (2009) Thermal, mechanical and rheological properties of poly (lactic acid)/epoxidized soybean oil blends. Polym Bull 62:91–98
Xiao HW, Lu W, Yeh JT (2009) Effect of plasticizer on the crystallization behavior of poly(lactic acid). J Appl Polym Sci 113:112–121
Zhang HL, Fang JY, Ge HH, Han LJ, Wang XM, Hao YP, Han CY, Dong LS (2013) Thermal, mechanical, and rheological properties of polylactide/poly(1,2-propylene glycol adipate). Polym Eng Sci 53:112–118
Ljungberg N, Wesslen B (2002) The effects of plasticizers on the dynamic mechanical and thermal properties of poly(lactic acid). J Appl Polym Sci 86:1227–1234
Li YJ, Shimizu H (2007) Toughening of polylactide by melt blending with a biodegradable poly(ether)urethane elastomer. Macromol Biosci 7:921–928
Zhang W, Chen L, Zhang Y (2009) Surprising shape-memory effect of polylactide resulted from toughening by polyamide elastomer. Polymer 50:1311–1315
Agrawal A, Saran AD, Rath SS, Khanna Ashok (2004) Constrained nonlinear optimization for solubility parameters of poly(lactic acid) and poly(glycolic acid)-validation and comparison. Polymer 45:8603–8612
Van Krevelen DW, Nijenhuis K (1955) Properties of polymers. Chapter 7:190–210
Marcilla A, Beltrán M (2004) Handbook of plasticizers. P. Chem. Tec Publishing, Toronto
Meng B, Deng JJ, Liu Q (2012) Transparent and ductile poly(lactic acid)/poly(butyl acrylate) (PBA) blends: structure and properties. Eur Polym J 48:127–135
Finkenstadt VL, Liu CK, Cooke PH, Liu LS, Willett JL (2008) Mechanical property characterization of plasticized sugar beet pulp and poly(lactic acid) green composites using acoustic emission and confocal microscopy. J Polym Environ 16:19–26
Coltelli MB, Maggiore ID, Bertoldo M, Signori F, Bronco S, Ciardelli F (2008) Poly(lactic acid) properties as a consequence of poly(butylene adipate-co-terephthalate) blending and acetyl tributyl citrate plasticization. J Appl Polym Sci 110:1250–1262
Jiang L, Wolcott MP, Zhang JW (2006) Study of biodegradable polyactide/poly(butylene adipate-co-terephthalate) blends. Biomacromolecules 7:199–207
Acknowledgments
This work was supported by the fund of Science & Technology Bureau of Jilin Province of China (No. 20126023) and the National High Technology Research and Development Program of China (863 Program) (No. 2012AA062904).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, H., Hao, Y., Liu, S. et al. Thermal, rheological, and mechanical properties of polylactide/poly(diethylene glycol adipate). Polym. Bull. 70, 3487–3500 (2013). https://doi.org/10.1007/s00289-013-1035-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-013-1035-8