Abstract
The purpose of this study is to investigate the use of nucleating agent to improve the overall performance of polybutylene adipate-co-terephthalate (PBAT) and calcium carbonate (CaCO3) composites. Specifically, sodium linoleate (Na-LL) is used as a chemical nucleating agent to improve the crystallization behavior of the material during melting extrusion processing. Mechanical, thermal, and morphological characteristics of PBAT and its composites were investigated. And a preliminary exploration was conducted on the mechanism of Na-LL improving the performance of PBAT. In this work, a formula has been obtained that dramatically saves production costs while improving product performance. After adding Na-LL to the composites, the thermal properties of the material were significantly improved, with a maximum crystallization temperature increase of 27.79 °C. In addition, composites offer superior mechanical properties compared to pure PBAT. When 0.2 parts by mass(pbw) Na-LL was added, the flexural modulus of the composites increased from 80.0 to 145.3 MPa, increased by 81.25%. The tensile performance can still be maintained at 11.0 Mpa, with elongation at break and impact strength reduced to 347.3% and 172.1 J/m, respectively. Under these conditions, it has excellent comprehensive mechanical properties. The results in this study suggest that Na-LL is a promising nucleating agent for enhancing the performance of composites and provides potential development of advanced materials for a wide range of applications in plastic packaging and plastic bags.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics are widely utilized in various industries, such as food packaging [1], agriculture [2]. These plastics are predominantly derived from petrochemical sources [3]. While it is undeniable that plastics has played an essential role in progress of society and industry, the recycling of plastic has become a highly significant environmental issue. Thus, creating a large volume of biodegradable plastics is considered one of the best techniques for addressing environmental concerns. Biodegradable plastics can be recycled or burnt, similar to conventional plastics produced from fossil fuels. However, unlike traditional plastics, they can be composted, enabling decomposition through microbial activity, and providing a feasible model for a zero-waste circular economy [4].
Currently, there are various types of degradable plastics that have been put into use in the market. Polylactic acid (PLA) is a new type of biodegradable material made from starch derived from renewable plant resources such as corn. Most biodegradable plastic straws on the market are made using PLA. It is also widely used in the pharmaceutical field, such as producing disposable infusion equipment, detachable surgical sutures, and so on [5]. As intracellular carbon and energy storage materials, polyhydroxyalkanoates (PHA) are a diverse biopolyesters synthesized by many bacteria [6]. Many studies have shown that PHA is a promising implant material due to its superior mechanical properties, biodegradability, and tissue compatibility [7]. Polybutylene succinate (PBS) is an aliphatic polyester synthesized from succinic acid and 1,4-butanediol (BDO) [8] and has been widely used in packaging, textiles, and agricultural plastic films.
PBAT, as an aromatic-aliphatic copolyster, is a typical example of biodegradable polymeric materials (BPMs) from petrochemical resources [9]. The mechanical and physical characteristics of PBAT are comparable to those of polyethylene [10]. With the necessary processing, it can replace most commonly used plastic products. Yet, high costs restrict the further application of PBAT in industries [11].
Introducing fillers into PBAT not only reduce the cost of the material, but also improves the performance of the product to a certain extent. In order to improve the performance, the polymer, starch, bamboo powder, and other natural fillers [12,13,14,15,16,17,18,19,20,21,22] are utilized as well as the mineral filler calcium carbonate (CaCO3) [23,24,25,26,27]. Polymer materials also post outstanding improvement effects on PBAT in order to compensate for some performance restrictions in industrial manufacturing. Through utilizing the benefits of other components to make up for the inadequacies of composites, it is an effective and forward-thinking modification strategy to enhance their performance [28,29,30,31,32,33,34,35].
CaCO3 is one of the most abundant materials on our planet and has been used for various polymer composites [36]. Despite this, due to poor interfacial compatibility between composites, the mechanical properties of the materials may deteriorate [37]. In actual production, additives are typically used to change the defect characteristics of composites. Commonly used additives include compatibilizers, chain extenders [12, 24]. Literature has confirmed that fillers added into PBAT composites play a role as nucleating agents to a certain extent, which can post effects on and change the performance of PBAT [17, 38, 39]. In actual production, there is still little research on nucleating agents used to modify PBAT. Therefore, it is necessary to explore the role of nucleating agents in the field of PBAT.
Sodium linoleate (Na-LL) is a widely used, reasonably priced long alkyl chain acid salt with excellent thermal stability that is obtained from biomass. As an efficient nucleating agent for polyester material PET, Na-LL can effectively promote its crystallization behavior through SN2 reaction [40]. Na-LL will react with ester chains in a molten state, which can destroy chain segments and attach an ionic group to the molecule. The ionic groups at the end of the molecular chain are prone to form stable crystal nuclei during the process, which can greatly shorten the nucleation cycle and improve the overall crystallization rate. Given the high similarity in chemical structure between PBAT and PET, it can hypothesize that Na-LL also exhibits a certain degree of efficacy in promoting the behavior of PBAT. Although Na-LL has an efficient effect, there is little research on it in polyester, so it has great research significance. For broader applications in engineering domains, this work looked thoroughly into the mechanism of action of nucleating, crystallization melting behavior, and mechanical properties of ternary composites.
Experimental
Materials
Polybutylene adipate-co-terephthalate (PBAT) (specific gravity = 1.24 g⋅cm−3) was supplied by JINHUI ZHAOLONG (Shanxi, China). Na-LL was supplied by Weng Jiang Reagent (Guangdong, China) and used as a nucleating agent with an average molecular weight of 304 g⋅mol−1. Shijiazhuang Tomalin Mineral Products Co., Ltd (Hebei, China) supplied the CaCO3 with a molecular weight of 100.19 g⋅mol−1. The average size of CaCO3 particles is 10 μm.
Preparation of PBAT/CaCO3/Na-LL ternary composites
Prior to use, the PBAT pellets and CaCO3 were dried in an oven at 110 °C for 6 h. Table 1 summarizes the composition of PBAT preparations and their ternary composites. The CaCO3 contents were chosen by following the literature, to ensure the service performance of materials as much as possible under the condition of meeting economic benefits [2, 26]. The amounts of PBAT and CaCO3 are fixed at 70 and 30 pbw, respectively. And add 0, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 pbw of Na-LL, respectively, for each group. All ingredients were well blended with PBAT before processing. The reactive extrusion was placed at molten zone of the twin-screw extruder. The temperature of screw 1–5 zone was set at 160, 170, 180, 185 and 185 °C, and the extruder head temperature and melt temperature were 180 °C and 170 °C, respectively.
Crystallization characteristics
By using a differential scanning calorimeter (TA Q2000, USA), the isothermal melt crystallization behavior of the PBAT composites was investigated. All DSC measurements were performed in a nitrogen environment, using an empty metal pan as a standard. The samples were first heated from 40 to 180 °C at a rate of 20 °C⋅min−1. To erase the thermal history, constant temperature was kept for 3 min. The melt was then cooled to 30 °C and heated to 180 °C at a rate of 10 °C⋅min.−1. Equation (1) below was used to determine the overall percent crystallinity (XC):
where \(\Delta {\text{H}}_{{\text{0}}}\), the melting enthalpy of 100% crystalline PBAT, is 114 J⋅g−1[41], and \({\text{W}}\%\) is the mass fraction of the PBAT matrix.
Mechanical properties test
According to ASTM test method, the mechanical properties of pure PBAT and their composites were measured with a microcomputer-controlled universal testing machine (WDT-W-60B1, China), including the tensile properties of D-638 and the bending properties of D-790. The impact performance is tested on the basis of D-256 using a Cantilever beam impact testing machine (HYU-XB-5, China). Due to the extremely high toughness of PBAT at room temperature, the impact strength of samples was tested after being stored at − 25 °C for 6 h to determine the low temperature impact strength. At least seven samples were tested for each group, the average value with standard deviation was obtained.
Morphology observation
The morphology of pure PBAT and their composites was characterized by scanning electron microscopy (JSM-IT500A, Japan), at an acceleration voltage of 10 kV. The samples were sputtered with gold to 2 nm thickness by spraying gold.
Fourier transform infrared spectroscopy (FTIR)
Identification of functional groups in the chemical structures of the materials was carried out by Fourier transform infrared spectroscopy (Thermo Scientific Nicolet iS20 spectrometer, USA). The spectra were recorded using attenuated total reflectance accessory, in the range of 500–4000 cm−1, and 64 scans were carried out.
Gel permeation chromatography (GPC)
Gel permeation chromatography was used to access the molecular weight of the samples (Waters 1515, USA). To prepare a 1 mg⋅mL−1 solution, 10 mg of totally dried material was thoroughly dissolved in 10 mL of tetrahydrofuran solution. To eliminate impurity, the produced solution was filtered using a filter membrane after being dispersed by ultrasonic for 30 min. The mobile phase was tetrahydrofuran, and the flow rate was 1 mL⋅min−1. Then, the molecular weight of samples was determined.
Results and discussions
The non-isothermal crystallization behaviors of PBAT and composites samples
The DSC results for PBAT, PBAT/CaCO3, and their composites are summarized in Table 2. According to the findings of the second heating scan of pure PBAT, the melting temperature (Tm) was 122.75 °C and the crystallization temperature (Tc) was 72.77 °C. The table demonstrates that CaCO3 as an inorganic nucleating agent can effectively increase the crystallization temperature of PBAT [42]. Figure 1(a) illustrates that addition of Na-LL raised the melting temperature (Tm) of composites, which will improve the ability of materials to perform heat sealing at high temperatures. CaCO3 was added to PBAT as a filler, and the addition of the filler can increase the physical barriers between polymer molecular chains. The presence of fillers increased the intersection of polymer chains, hinders the movement of molecules, and thus increased the melting temperature of the polymer [43]. PBAT caused ordered arrangement of polymer molecules under the crystallization of Na-LL and melting in this stable and ordered structure requires higher temperatures. The melting enthalpies (Hm) of samples decreased due to the addition of CaCO3. It was clear that CaCO3 can influence the melting enthalpy of PBAT. As a filler, CaCO3 can change the density and thermal capacity of composites. At the same time, CaCO3 itself also has a certain thermal capacity, which can influence the overall thermal capacity of the composites and will cause a change in the melting enthalpy of PBAT. The inclusion of Na-LL can significantly enhance the phenomenon of crystalline fraction decrease caused by the addition of CaCO3 and boost the crystallization temperature of the composites because Na-LL may be utilized in composites as a heterogeneous nucleus.
From the perspective of chemical nucleation, organic carboxylic acid sodium salts may react with ester chains, while PBAT is still in a molten state. And nucleating agents may react with PBAT molecular segments, resulting in a decrease in the molecular weight of the composites. This effect helps to shorten the nucleation cycle and accelerate the overall crystallization rate of the composites. In addition, the increase in comprehensive crystallization temperature and crystallization rate indicate that Na-LL post a positive impact on the thermal properties of PBAT [44].
Mechanical properties
The impacts from Na-LL on the mechanical properties of PBAT and their composites are investigated and demonstrated in Table 3 and Fig. 2. Firstly, the mechanical characteristics of PBAT are significantly affected by the addition of CaCO3 filler, particularly in terms of stiffness. And the flexural modulus of the composites has been improved in accordance with its effect. The hydrophobicity of PBAT, on the other hand, makes it incompatible with hydrophilic CaCO3, and the poor dispersion effect of CaCO3 in the PBAT matrix caused a significant decrease in the tensile strength of the composites [16, 45]. It also decreased in the elongation at break and impact strength of P7/C3 samples.
It also can be seen from the table that the flexural modulus of samples added with Na-LL has been effectively enhanced compared with PBAT/CaCO3 composites. This can be attributed to the effect of Na-LL in the PBAT matrix, which reduces the molecular weight of PBAT and facilitates better dispersion of CaCO3 in the PBAT matrix [46]. However, excessive use of Na-LL can result in increasing brittleness of the composites. The addition of too much Na-LL also contributes to better dispersion of CaCO3 in the matrix and increases flowability of the entire system. Although this phenomenon can enhance the dispersion of CaCO3 in the matrix, it may make stress unable to conduct effectively and increase brittleness of the composites. Therefore, the optimal quantity of Na-LL must be determined for enhancing the dispersibility of CaCO3 in the matrix while maintaining the mechanical properties of the composites. Meanwhile, Na-LL itself can also cause chemical degradation of PBAT, which is one of the reasons why the tensile strength of PBAT composites is decreasing [40].
It is worth noting that compared to the P7/C3 sample, the tensile strength and elongation at break of the composites are marginally increased with the addition of 0.05 pbw Na-LL. It may be that at low concentration, Na-LL has a greater dispersion in the matrix and can enhance the distribution of various components in the matrix following the interaction with PBAT. This action can enable stress to be dispersed to other areas during stress, effectively preventing stress concentration. When 0.2 pbw Na-LL was added, the flexural modulus of the composites increased from 80.0 to 145.3 MPa. It may not only increase flexural modulus by 81.25%, but also guarantee the original toughness, ensuring the product to achieve the best overall mechanical qualities.
Morphological analysis
SEM was utilized to investigate the microscopic morphology from the impact sections of PBAT and their composites (2000 ×), as exhibits in Fig. 3 was obtained. The impact fracture cross-section of pure PBAT is shown in Fig. 3(a). The stretched matrix on the microscopic surface of the PBAT sample is due to the ductile fracture. This is because PBAT itself has excellent toughness and ductility. The absorbed stress may be efficiently distributed in the matrix and can resist more impact when exposed to impact energy. It can also be explained by the existence of a thicker PBAT matrix near the fracture contact, which is able to resist greater stress effects [47].
As CaCO3 is disseminated in the PBAT matrix, the impact strength reduces when exposed to stress impact. As shown in Fig. 3(b), due to the effect of stress, the fracture mode of the composites is still ductile fracture. The observation of a filamentary matrix on the fracture surface of the PBAT matrix can indicate that the PBAT/CaCO3 composites are more prone to fracture at this stage. This is a result of the distribution of dispersed CaCO3 in PBAT and the irregular aggregation of CaCO3 into large particles, which leads to poor PBAT continuity. While under stress, it is challenging to transfer the tension to different places.
Based on the P7/C3 system, the incorporation of Na-LL has brought about significant changes in the impact fracture surface of composites. The interaction between Na-LL and PBAT reduces the molecular weight of PBAT, improving the fluidity of the material. This phenomenon also enhances the dispersion of CaCO3 in the PBAT matrix, resulting in a more uniform and stable structure. As a result, the fracture behavior of the composites began to shift from ductile to brittle. The Fig. 3(c) shows that after being subjected to an impact fracture, the composites exhibit a uniform and pure surface, accompanied by small holes and voids. These voids are believed to be the result of brittle fracture behavior. The increase in brittleness of the composites is attributed to the improved dispersion of CaCO3 particles in the PBAT matrix by the addition of Na-LL. The improvement of dispersion leads to the formation of a more uniform and stable structure, which also makes the sample more prone to separation when subjected to impact. Thus, the impact strength of composites has further decreased.
Discussion on action mechanism of Na-LL in PBAT/CaCO3
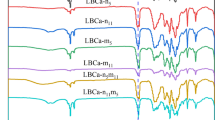
After FTIR characterization of the sample, the most representative characterization results were selected and shown in Fig. 4. The spectrum of PBAT shows typical characteristic aromatic and aliphatic peaks, which is in line with what would be expected given the chemical composition of material. The main peaks of PBAT are 2951, 2867, 1401, 1358, 1169, 1108 and 937 cm−1. 1169 and 1108 cm−1 are the left–right symmetrical tensile vibration absorption of C–O; 1018 cm−1 is the bending vibration reduction in phenylene ring –CH–; 937 cm−1 is carboxylic acid group -C–OH bending vibration; 2951 and 2867 cm−1 are methylene asymmetric stretching; 1401 cm−1 is CH2-plane bending vibration; 1358 cm−1 is out-of-plane bending of CH2; Four or more neighboring CH2- groups make up the peaks at 728 cm−1. The benzene metabolite curve peak is 700–900 cm−1 [24, 48].
The C-H bond symmetric vibrational absorption of CH2 and CH3 in the Na-LL carbon chain is represented by the Na-LL absorption peaks at wave numbers of 2925 and 2850 cm−1, respectively. 1565 cm−1 belongs to the characteristic absorption peak of –COO– group [40]. Comparing the spectra of pure PBAT and P/0.2N, the relevant characteristic peak of CH2 group in PBAT has shifted, which may be caused by the reaction between Na-LL and carboxyl and hydroxyl groups in PBAT matrix. PBAT is a polyester polymer material with the basic components of adipic acid and butanediol ester, and the added of Na-LL may interact with the carbonyl or ester group in PBAT molecule. Once Na-LL is added, the molecular structure of PBAT may be changed. This structural change may lead to changes in the vibrational frequencies of some chemical bonds in the PBAT molecule, resulting in peak intensity reduction near 1260 and 1710 cm−1. The C-O exhibits symmetric stretching vibrations at 1169 and 1108 cm−1.
Notably, it can be found that the stretching vibrations of C-O at 1169 and 1108 cm−1 blue shift to 1093 and 1042 cm−1 locations, respectively, after adding nucleating agent. The reaction shown in Fig. 5 may occur, which changes the vibration frequency of the C-O bond and causes that C-O stretching vibration peak shift toward a lower wavenumber direction.
Comparing the spectra of pure P/0.2N and P7/C3/0.2N samples, CaCO3 fillers, PBAT and Na-LL composites may interact through physical adsorption and chemical reactions, among other mechanisms, resulting in a weaker peak strength and ester bond interaction strength. In addition, the PBAT matrix and the CaCO3 component could interact. These interactions make Na-LL disperse more evenly throughout the whole matrix, which improves the effectiveness of Na-LL [24]. The molecular structure of the composites may change as a result of these interactions, diminishing or eliminating some distinctive peaks. The spectral signals in the composites may also be attenuated or scattered by the CaCO3 fillers, which contributed to a drop in signal strength close to the spectral peak. When too much filler is used, this phenomenon is more noticeable.
The mechanical properties of polymers are closely related to their molecular weight (MW). The MW data obtained from testing pure PBAT, P7/C3, P/0.2N, and P7/C3/0.2N samples are shown in Table 4. Compared to pure PBAT, the addition of CaCO3 resulted in an increase in the molecular weight of polymer, which can be explained by the interaction of PBAT and CaCO3 during melt extrusion. In the molten state, PBAT molecular chains undergo intense movement and cannot be arranged in an orderly manner. CaCO3 filler causes PBAT molecular chains to entangle, leading to an increase in the molecular weight of the composites [43]. The addition of the nucleating agent caused a decrease in the molecular weight of PBAT, which may be due to the effect of Na-LL on the chemical nucleation of polymer, leading to chain breakage. However, the addition of CaCO3 can compensate for this defect in the composites and help maintain its properties. It explains the changes in peak intensity observed in the characterization of infrared in the preceding text and also suggests the mechanism by which nucleating agent acted on PBAT.
Conclusions
In light of the comprehensive analysis of mechanical and thermal properties, it is believed that it is feasible to use Na-LL as a nucleating agent to improve the rigidity and crystallinity of PBAT-based composites. When 0.2 pbw Na-LL was added, the flexural modulus of the composites increased from 80 to 145 MPa, and other mechanical properties were well maintained. Under the combined action of CaCO3 and Na-LL, the crystallization temperature of the samples increased from 72.77 ℃ to 91.02 ℃. A formula has been obtained that greatly saves production costs while improving the performance of the product by using the nucleating agent. Although using the nucleating agent can cause chemical degradation in PBAT and influence its molecular weight, the addition of CaCO3 compensates for this effect. According to GPC analysis, the molecular weight of the modified PBAT remains equivalent to that of pure PBAT, thus ensuring that the comprehensive performance of the composites is not negatively affected. Therefore, Na-LL also provides a reliable modification pathway for the application of PBAT composites.
References
Verdi AG, De Souza AG, Rocha DB, De Oliveira SA, Alves RMV, Dos Santos RD. Biodegradable films functionalized with Moringa oleifera applied in food packaging. Iran Polym J. 2020;30(3):235–46.
Yang Y, Zhang C, Weng Y. Effects of CaCO3 surface modification and water spraying on the weathering properties of PBAT/CaCO3 films. Polym Test. 2021;102:107334.
Iwata T. Biodegradable and bio-based polymers: future prospects of eco-friendly plastics. Angew Chem Int Ed. 2015;54(11):3210–5.
Rai P, Mehrotra S, Priya S, Gnansounou E, Sharma SK. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour Technol. 2021;325:124739.
Ebrahimi F, Ramezani DH. Poly lactic acid (PLA) polymers: from properties to biomedical applications. Int J Polym Mater Polym Biomater. 2021;71(15):1117–30.
Sehgal R, Gupta R. Polyhydroxyalkanoate and its efficient production: an eco-friendly approach towards development. 3 Biotech. 2020;10(12):549.
Zhang J, Shishatskaya EI, Volova TG, da Silva LF, Chen GQ. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater Sci Eng C. 2018;86:144–50.
Kantor-Malujdy N, Skowron S, Michalkiewicz B, El Fray M. Poly (butylene-succinate)-based blends with enhanced oxygen permeability. Mater Today Commun. 2022;33:104306.
Witt U, Yamamoto M, Seeliger U, Müller R-J, Warzelhan V. Biodegradable polymeric materials—Not the origin but the chemical structure determines biodegradability. Angew Chem Int Ed. 1999;38(10):1438–42.
Liu J, Wang P, Wang Y, Zhang Y, Xu T, Zhang Y, Xi J, Hou L, Li L, Zhang Z, Lin Y. Negative effects of poly (butylene adipate-co-terephthalate) microplastics on Arabidopsis and its root-associated microbiome. J Hazard Mater. 2022;437:129294.
Yang F, Chen G, Li J, Zhang C, Ma Z, Zhao M, Yang Y, Han Y, Huang Z, Weng Y. Effects of quercetin and organically modified montmorillonite on the properties of poly(butylene adipate-co-terephthalate)/thermoplastic starch active packaging films. ACS Omega. 2023;8(1):663–72.
Olivato J, Grossmann M, Yamashita F, Eiras D, Pessan L. Citric acid and maleic anhydride as compatibilizers in starch/poly (butylene adipate-co-terephthalate) blends by one-step reactive extrusion. Carbohyd Polym. 2012;87(4):2614–8.
Garcia PS, Grossmann MV, Shirai MA, Lazaretti MM, Yamashita F, Muller CM, Mali S. Improving action of citric acid as compatibiliser in starch/polyester blown films. Indus Crops Products. 2014;52:305–12.
Kim JH, Lee JC, Kim GH. Study on poly (butylene adipate-co-terephthalate)/starch composites with polymeric methylenediphenyl diisocyanate. J Appl Polym Sci. 2015;132(16):41884.
Wei D, Wang H, Xiao H, Zheng A, Yang Y. Morphology and mechanical properties of poly (butylene adipate-co-terephthalate)/potato starch blends in the presence of synthesized reactive compatibilizer or modified poly (butylene adipate-co-terephthalate). Carbohydrate Polym. 2015;123:275–82.
Moustafa H, Guizani C, Dufresne A. Sustainable biodegradable coffee grounds filler and its effect on the hydrophobicity, mechanical and thermal properties of biodegradable PBAT composites. J Appl Polym Sci 2017; 134(8):44498
Garalde RA, Thipmanee R, Jariyasakoolroj P, Sane A. The effects of blend ratio and storage time on thermoplastic starch/poly(butylene adipate-co-terephthalate) films. Heliyon. 2019;5(3):e01251.
Xiong S-J, Pang B, Zhou S-J, Li M-K, Yang S, Wang Y-Y, Shi Q, Wang S-F, Yuan T-Q, Sun R-C. Economically competitive biodegradable PBAT/lignin composites: effect of lignin methylation and compatibilizer. ACS Sustain Chem Eng. 2020;8(13):5338–46.
Zhai X, Wang W, Zhang H, Dai Y, Dong H, Hou H. Effects of high starch content on the physicochemical properties of starch/PBAT nanocomposite films prepared by extrusion blowing. Carbohydrate Polym. 2020;239:116231.
Liu Y, Liu S, Liu Z, Lei Y, Jiang S, Zhang K, Yan W, Qin J, He M, Qin S, Yu J. Enhanced mechanical and biodegradable properties of PBAT/lignin composites via silane grafting and reactive extrusion. Compos Part B: Eng. 2021;220:108980.
Li W, Huang J, Liu W, Qiu X, Lou H, Zheng L. Lignin modified PBAT composites with enhanced strength based on interfacial dynamic bonds. J Appl Polym Sci. 2022;139(27):e52476.
Xie L, Huang J, Xu H, Feng C, Na H, Liu F, Xue L, Zhu J. Effect of large sized reed fillers on properties and degradability of PBAT composites. Polym Compos. 2023;44(3):1752–61.
Wang XW, Wang GX, Huang D, Lu B, Zhen ZC, Ding Y, Ren ZL, Wang PL, Zhang W, Ji JH. Degradability comparison of poly (butylene adipate terephthalate) and its composites filled with starch and calcium carbonate in different aquatic environments. J Appl Polym Sci. 2019;136(2):46916.
Nunes EDC, De Souza AG, Rosa DDS. Use of a chain extender as a dispersing agent of the CaCO3 into PBAT matrix. J Compos Mater. 2020;54(10):1373–82.
Titone V, La Mantia FP, Mistretta MC. The effect of calcium carbonate on the photo-oxidative behavior of poly (butylene adipate-co-terephthalate). Macromol Mater Eng. 2020;305(10):2000358.
Zhang T, Zhang C, Yang Y, Yang F, Zhao M, Weng Y. Improved properties of poly (butylene adipate-co-terephthalate)/calcium carbonate films through silane modification. J Appl Polym Sci. 2021;138(38):50970.
Rapisarda M, Mistretta MC, Scopelliti M, Leanza M, La Mantia FP, Rizzarelli P. Influence of calcium carbonate nanoparticles on the soil burial degradation of Polybutyleneadipate-Co-Butylenetherephthalate films. Nanomaterials. 2022;12(13):2275.
Jarapanyacheep R, Ruksakulpiwat Y, Jarukumjorn K. Melt processing of maleic anhydride grafted poly (lactic acid) and its compatibilizing effect on poly (lactic acid)/poly (butylene adipate-co-terephthalate) blend and their composite. Polym Sci Ser A. 2017;59:384–96.
Costa AR, Reul LT, Sousa FM, Ito EN, Carvalho LH, Canedo EL. Degradation during processing of vegetable fiber compounds based on PBAT/PHB blends. Polym Test. 2018;69:266–75.
Rocha DB, Souza De Carvalho J, De Oliveira SA, Dos Santos Rosa D. A new approach for flexible PBAT/PLA/CaCO3 films into agriculture. J Appl Polym Sci 2018; 135(35): 46660.
Balaji S, Venkatesan R, Mugeeth L, Dhamodharan R. Hydrophobic nanocomposites of PBAT with Cl-fn-POSS nanofiller as compostable food packaging films. Polym Eng Sci. 2021;61(1):314–26.
Pal AK, Wu F, Misra M, Mohanty AK. Reactive extrusion of sustainable PHBV/PBAT-based nanocomposite films with organically modified nanoclay for packaging applications: compression moulding vs cast film extrusion. Compos Part B: Eng. 2020;198:108141.
Zhao H, Liu H, Liu Y, Yang Y. Blends of poly (butylene adipate-co-terephthalate) (PBAT) and stereocomplex polylactide with improved rheological and mechanical properties. RSC Adv. 2020;10(18):10482–90.
Tian H-L, Wang Z-P, Jia S-L, Pan H-W, Han L-J, Bian J-J, Li Y, Yang H-L, Zhang H-L. Biodegradable foaming material of poly(butylene adipate-co-terephthalate) (PBAT)/Poly(propylene carbonate) (PPC). Chin J Polym Sci. 2021;40(2):208–19.
Wu D, Guo Y, Huang A, Xu R, Liu P. Effect of the multi-functional epoxides on the thermal, mechanical and rheological properties of poly (butylene adipate-co-terephthalate)/polylactide blends. Polym Bull. 2021;78(10):5567–91.
Shi N, Cai J, Dou Q. Crystallization, morphology and mechanical properties of PLA/PBAT/CaCO3 composites. Adv Mater Res. 2013;602:768–71.
Wongphan P, Nerín C, Harnkarnsujarit N. Enhanced compatibility and functionality of thermoplastic cassava starch blended PBAT blown films with erythorbate and nitrite. Food Chem. 2023;420:136107.
Fukushima K, Wu M-H, Bocchini S, Rasyida A, Yang M-C. PBAT based nanocomposites for medical and industrial applications. Mater Sci Eng, C. 2012;32(6):1331–51.
Wang Y, Liu Q, Zhen ZC, Liu JL, Qiao RM, He WQ. Effects of mica modification with ethylene-vinyl acetate wax on the water vapor barrier and mechanical properties of poly-(butylene adipate-co-terephthalate) nanocomposite films. J Appl Polym Sci. 2021;138(26):50610.
Huang WT, He GJ, Tang WD, Zou XL, Yin XC. A facile approach to realize simultaneously chain extension and crystallization promotion of poly (ethylene terephthalate). Polym Degrad Stab. 2021;190:109644.
Costa AR, Almeida TG, Silva SM, Carvalho LH, Canedo EL. Chain extension in poly (butylene-adipate-terephthalate). Inline testing in a laboratory internal mixer. Polym Test. 2015;42:115–21.
Gou J, Zhang L, Li C. A new method combining modification of montmorillonite and crystal regulation to enhance the mechanical properties of polypropylene. Polym Test. 2020;82:106236.
Nunes EDC, Souza AGD, Coiado RD, Moura EA, Rosa DDS. Evaluation of the poly (lactic acid) and calcium carbonate effects on the mechanical and morphological properties in PBAT blends and composites. Int J Innov Sci Eng Technol. 2017;4(6):313–8.
Liu Y, Wirasaputra A, Jiang Z, Liu S, Zhao J, Fu Y. Fabrication of improved overall properties of poly (ethylene terephthalate) by simultaneous chain extension and crystallization promotion. J Therm Anal Calorim. 2018;133(3):1447–54.
Pan H, Ju D, Zhao Y, Wang Z, Yang H, Zhang H, Dong L. Mechanical properties, hydrophobic properties and thermal stability of the biodegradable poly(butylene adipate-co-terephthalate)/maleated thermoplastic starch blown films. Fibers and Polymers. 2016;17(10):1540–9.
Chen RY, Zou W, Zhang HC, Zhang GZ, Yang ZT, Jin G, Qu JP. Thermal behavior, dynamic mechanical properties and rheological properties of poly (butylene succinate) composites filled with nanometer calcium carbonate. Polym Test. 2015;42:160–7.
Venkatesan R, Sivaprakash P, Kim I, Eldesoky GE, Kim S-C. Tannic acid as a crosslinking agent in poly (butylene adipate-co-terephthalate) composite films enhanced with carbon nanoparticles: processing, characterization, and antimicrobial activities for food packaging. J Environ Chem Eng. 2023;11(4):110194.
Sirisinha K, Somboon W. Melt characteristics, mechanical, and thermal properties of blown film from modified blends of poly (butylene adipate-co-terephthalate) and poly (lactide). J Appl Polym Sci. 2012;124(6):4986–92.
Acknowledgements
This work was financially supported by Natural Science Foundation of Changsha (No. kq2202184) and Hunan Provincial Science and Technology Department (No. 2021GK5007).
Author information
Authors and Affiliations
Contributions
SS was contributed to methodology, data curation, writing—original draft, visualization, investigation. ZL was contributed to writing—review and editing, visualization. YL was contributed to writing—original draft, investigation. YL was contributed to validation, supervision. J-RZ was contributed to supervision, writing—review and editing. JT was contributed to supervision, writing—review and editing. Y-FZ was contributed to project administration, funding acquisition, supervision, writing—review and editing, conceptualization.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sheng, S., Li, Z., Li, Y. et al. Using sodium linoleate as a nucleating agent to improve the properties of PBAT/CaCO3 composites. J Therm Anal Calorim 148, 13859–13868 (2023). https://doi.org/10.1007/s10973-023-12584-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12584-5