Abstract
Recent research has revealed the successful use of thermogravimetric analysis (TGA) in dynamic mode as an alternative method for determining degrees of oil conversion into biodiesel. This paper reports the results obtained with the use of TGA in quasi-isothermal mode (TGA-qISO) and with quantitative proton nuclear magnetic resonance (1H qNMR) to investigate the compositions of blends of soybean oil and biodiesels (10:0, 8:2, 6:4, 4:6, 2:8, and 0:10, m:m). Also, reported are data acquired by applying a programmed sequence of TGA-qISO and gas chromatography with flame ionization detection (GC-FID) steps to a biodiesel witness sample and samples prepared by a partner laboratory. All the biodiesels were obtained by transesterification via the methyl route using homogeneous basic catalysis. Thermogravimetric curves (TG) clearly distinguished between mass losses caused by ester volatilization and those resulting from oil thermal decomposition. Ester contents calculated by TGA-qISO proved concordant with 1H qNMR and GC-FID data, with maximum differences of around 0.5% and 1.4%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fossil fuels are the most crucial resources for the world’s current energy supply [1]. Since burning these fuels generates greenhouse gases that contribute to climate change [2], alternative, renewable energy sources have attracted increasing interest, to minimize or even replace fossil fuels [3]. Biodiesels are low-toxicity, biodegradable biofuels devoid of sulfur or aromatic compounds. They produce less exhaust emissions, owing to high flash points, and can be obtained from a wide range of raw materials, including vegetable, seaweed, and microbial oils, animal fats, and even waste oils [4,5,6].
Biodiesel synthesis, however, generates contaminants such as intermediate glycerols, non-transesterified triglycerides, free fatty acids, and residual alcohols, which can pose serious operational problems, including deposit formation in engines, filter clogging, and fuel deterioration [7]. Quality requirements for biofuel commercialization include determining the degree of conversion of oil into biodiesel [8], using gas chromatography with flame ionization detection (GC-FID) [9]. This method, however, requires sample preparation with organic solvents such as n-heptane, toluene, and hexane—highly toxic and potentially polluting substances [9, 10].
Thermogravimetric analysis (TGA), although widely used to investigate biodiesel properties, including thermal stability, oxidation time, activation energy, and purity, has been employed to this end in dynamic heating mode, whereby mass loss is continuously recorded as the temperature is linearly increased [11,12,13,14,15,16,17]. This approach, however, may fail to distinguish between overlapping thermal decomposition and volatilization events, yielding significant ester measurement differences relative to the standard method [17, 19]. In quasi-isothermal thermogravimetric analysis (TGA-qISO), instead, a programmed sequence of furnace temperature modes (dynamic, isothermal, dynamic) is followed for data acquisition. Initial furnace temperature is linearly increased (dynamic mode) until mass loss does not exceed a pre-defined value, at which point the furnace operates in isothermal mode until mass stabilizes, subsequently resuming dynamic mode until a TG curve has been fully plotted [18]. The present investigation compared ester contents in blends of soybean oil and biodiesel as quantified by TGA-qISO and 1H qNMR. In addition, values obtained using the programmed furnace operation sequence were compared against GC-FID data for samples prepared by a partner laboratory and a witness biodiesel sample.
Materials
Soybean oil (food grade) was purchased locally. Anhydrous methanol (Neon), potassium hydroxide (Neon), methyl heptadecanoate (used as an internal standard to quantify ester content by GC-FID; from Supelco), deuterated chloroform (CDCl3; Aldrich), C12H10 (biphenyl, as an internal standard; Merck), and tetramethylsilane (TMS; CIL) were also employed.
Biodiesels will be abbreviated as follows (drawing on the first letter of the oilseed genus concerned):
Glycine max (soybean): | BdG |
|---|---|
Scheelea phalerata (“bacuri”): | BdS |
Terminalia catappa (“sete-copas”): | BdT |
Experimental
Methyl biodiesel generation from uncombined soybean oil and soybean oil–biodiesel blends
The transesterification reaction was performed as per Verma and Sharma [19] on a 500 g oil mass at an oil-to-methanol molar ratio of 1:6. Reaction time was 1 h and the amount of catalyst 1% of the oil mass.
Blends of soybean oil and BdG were prepared at well-defined ratios (10:0, 8:2, 6:4, 4:6, 2:8, and 0:10, m:m) to better evidence correlations with mass losses on TGA-qISO curves. To this end, a mass corresponding to the established soybean oil content for each blend was weighed and a sufficient amount of biodiesel was added to complete a total mass of 10 g.
Biodiesel generation from Scheelea phalerata and Terminalia catappa
Biodiesels obtained from two oilseeds—Scheelea phalerata and Terminalia catappa—were kindly provided by partner researchers at the Chemical Technology Development Center of Universidade Estadual de Mato Grosso do Sul at Naviraí. These biodiesels had previously been investigated for thermal and oxidative stability in blends with soybean oil [20].
Ester quantification using GC-FID
Chromatography was performed according to the EN-1410 standard [21] in a GC 2010 gas chromatograph (Shimadzu) with an AOC-5000 sampler, equipped with a flame ionization detector. Stabilwax capillary columns (30 m; 0.25 mm; 0.25 µm) were used. Helium was the carrier gas, at a flow rate of 2.2 mL min–1. Sample volume was 1 µL, injected at a split ratio of 1:50.
Both injector and detector temperatures were maintained at 250 °C. Furnace temperature started at 60 °C (2 min isothermal regime), rising by 10 °C min–1 to 200 °C (followed by a 10 min isotherm), then by 5 °C min–1 to 240 °C (followed by a 7 min isotherm), comprising a total run time of 41 min. A methyl heptadecanoate solution at 10.02 mg mL–1 was prepared. A 50 mg mass from each sample was diluted in 1 mL of the internal standard solution.
Quantitative proton nuclear magnetic resonance spectroscopy (1H qNMR)
Samples for 1H qNMR analysis were prepared from 250 μL of blends of soybean oil and BdG, 250 μL of CDCl3,, and 15 mg of C12H10 (internal standard). The resulting solution was transferred to a NMR tube (5 mm) and TMS vapor was added. The samples were prepared in triplicate.
1H qNMR spectra were acquired at 20 °C on a 11.75 T Avance NEO 500 instrument (Bruker) equipped with a 5 mm multinuclear BBF Smart probe, observing 1H at 500.13 MHz. Spectra were obtained applying a 30° excitation pulse (zg30) with 16 scans, collecting 32 k points covering a spectral width of 23.8 ppm. Relaxation delay and acquisition time were 4 and 2 s, respectively, to guarantee a relaxation time longer than 7T1. NMR data were processed by applying an exponential multiplication by a factor of 0.3 Hz in free induced decay, and baseline and phase were manually corrected. All 1H qNMR spectra were referred at 0.00 ppm to the TMS signal.
The integrals of signals were automatically calculated. The α-glycerol signal at 4.38–4.06 ppm (4H) for soybean oil, the methyl signal at 3.80–3.40 ppm (3H, OCH3 group) for biodiesel, and the o-hydrogens from the internal standard (biphenyl) at 7.67–7.48 ppm (4H) were used for quantification. Ester content was calculated using the following equation [22]. 1H qNMR results were expressed in terms of biodiesel content (%, m:m).
In this equation, mx is the analyte mass (soybean oil or biodiesel); Ix and Istd are the integrated signal areas of analytes and internal standard, respectively; Nx and Nstd are the number of protons in the signals from analytes and internal standard, respectively; Mx and Mstd are the molar masses of analytes and internal standard, respectively; and Pstd and mstd are the purity and weighed mass of the internal standard, respectively.
Quasi-isothermal thermogravimetric analysis
TGA-qISO curves for soybean oil blended with BdG, BdS, or BdT were acquired on a TGA-Q50 device (TA Instruments) under synthetic air and nitrogen atmospheres, at a flow rate of 60 mL min–1 in the furnace and a heating ratio of 10 °C min–1, using platinum crucibles as support and sample masses of 5.5 ± 2 mg.
Data acquisition proceeded as follows, both for blends and uncombined samples:
-
stabilizing furnace temperature at 25 °C;
-
conditional rule: interrupt next step if mass variation exceeds 2%;
-
heating ramp at 10 °C min–1 to 350 °C;
-
conditional rule: interrupt next step if mass variation exceeds 2%;
-
isothermal regime for 70 min or while mass change does not reach 2%;
-
heating ramp at 10 °C min–1 to 650 °C.
Results and discussion
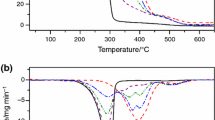
Figure 1 depicts the TGA-qISO curves for blends of soybean oil and BdG acquired under oxidizing and inert atmospheres.
Three or four mass loss stages can be observed in the curves plotted under synthetic air. The first stage (100–200 °C) was attributed to biodiesel volatilization, while subsequent stages (250–600 °C) were attributed to triglyceride thermal decomposition [16, 23,24,25]. The two or three mass loss stages, visible in the curves acquired under a nitrogen atmosphere, were attributed to biodiesel volatilization (100–200 °C) and triglyceride thermal decomposition (350–400 °C) [26]. The curves reveal that the stages of oil thermal decomposition in the blends manifest at higher temperatures when nitrogen is the carrier gas, minimizing the likelihood of overlapping events such as partial triglyceride decomposition and biodiesel volatilization. However, even with shorter temperature intervals between stages, the results obtained for synthetic air also proved accurate.
Table 1 shows the mass losses taking place under synthetic air and nitrogen atmospheres in the 100–200 °C range and respective biodiesel contents, as quantified by 1H qNMR. In this temperature range, no mass losses were detected for uncombined soybean oil under either atmosphere. Oil thermal decomposition started at 259 °C under synthetic air and 305 °C under nitrogen.
Figure 2 depicts analytical curves acquired under oxidizing and inert atmospheres, plotting mass losses (%) in the 100–200 °C range against biodiesel concentrations in the blends.
Table 2 shows linear regression data obtained under oxidizing and inert atmospheres.
Correlation coefficients exceeded 0.99 for both atmospheres, indicating a strong correlation between mass loss and biodiesel concentration in the blends [27].
To evaluate the efficiency of the programmed stepwise sequence, TGA-qISO curves were plotted for two biodiesel samples (BdS, BdT) prepared by a partner laboratory and for a BdG witness sample [16].
Table 3 depicts the methyl ester compositions of BdG, BdS, and BdT, as quantified by GC-FID.
BdS methyl esters were predominantly saturated (61.8%, m:m, comprising 58.2% having 8 to 16-carbon chains). Unsaturated methyl esters accounted for 37.6% (m:m). For BdT, saturated and unsaturated contents were 37.8% and 60.2% (m:m), respectively, while for BdG these ratios were 14.8% and 82% (m:m), respectively (all esters with 16 to 18-carbon chains). Correlated with the TGA-qISO curves, these compositions revealed well-defined mass losses.
Figure 3 shows the TGA-qISO curves obtained for BdG, BdS, and BdT under oxidizing and inert atmospheres.
For BdT and BdG, however, a single mass loss step was detected under both atmospheres (133 and 134 °C for synthetic air; 133 and 131 °C for nitrogen, respectively)—a loss attributed to volatilization of saturated and unsaturated methyl esters (C16:0–C18:3). These results were corroborated by GC-FID (Table 3). Two volatilization steps were detected for BdS, irrespective of atmosphere. The first step (at roughly 96 °C), involved 56.75% mass loss, attributed to volatilization of shorter-chained (C8:0–C16:0) saturated methyl esters. The second step (at roughly 96–197 °C) involved 42.48% mass loss, attributed to longer-chained saturated (C18) and unsaturated (C16–C18) esters.
Table 4 compares the ester contents in BdS, BdT, and BdG quantified by GC-FID and by TGA-qISO under oxidizing and inert atmospheres.
Small differences, in the 0.50–1.43% range, were found between techniques (Table 4). These differences were significantly smaller than those reported elsewhere [16, 28]. However, published data show that roughly 1.2% of soybean oil biodiesel mass is constituted of 20-, 21-, and 22-carbon fatty acids [15], which were not detected in the present GC-FID analysis.
Figure 4 depicts mass loss curves (%) as a function of time (min) plotted for BdG samples (roughly 5.0 mg) subjected to TGA-qISO under a nitrogen atmosphere, revealing that 70 min of isothermal regime proved sufficient time for full thermal decomposition of biodiesel, allowing ester content to be accurately quantified.
Conclusions
The results obtained revealed TGA-qISO conducted with stepwise programming to be an accurate, reproducible method for quantifying ester content in biodiesels, with a maximum difference of 1.43% relative to the EN-14103 gas chromatography standard—a much smaller difference than that obtained with TGA conducted in dynamic heating mode [13, 16, 28]. Given its lower cost (requiring no pre-treatment of samples with toxic solvents), efficiency, and fast application, the proposed method has the potential to be employed as an alternative to official standards for biodiesel quality control.
References
Gebremariam SN, Marchetti JM. Economics of biodiesel production: review. Energy Convers Manag. 2018. https://doi.org/10.1016/j.enconman.2018.05.002.
Ito K. CO2 emissions, renewable and non-renewable energy consumption, and economic growth: evidence from panel data for developing countries. Int Econ. 2017. https://doi.org/10.1016/j.inteco.2017.02.001.
Teo SH, Islam A, Taufiq-Yap YH. Algae derived biodiesel using nanocatalytic transesterification process. Chem Eng Res Des. 2016. https://doi.org/10.1016/j.cherd.2016.04.012.
Zhang W-B. Review on analysis of biodiesel with infrared spectroscopy. Renew Sustain Energy Rev. 2012. https://doi.org/10.1016/j.rser.2012.07.003.
Ambat I, Srivastava V, Sillanpää M. Recent advancement in biodiesel production methodologies using various feedstock: a review. Renew Sustain Energy Rev. 2018. https://doi.org/10.1016/j.rser.2018.03.069.
Abed KA, Gad MS, El Morsi AK, Sayed MM, Elyazeed SA. Effect of biodiesel fuels on diesel engine emissions. Egypt J Pet. 2019. https://doi.org/10.1016/j.ejpe.2019.03.001.
Knothe G. Analyzing biodiesel: standards and other methods. JAOCS J Am Oil Chem Soc. 2006. https://doi.org/10.1007/s11746-006-5033-y.
Lôbo IP, Ferreira LSC. Biodiesel: parâmetros de qualidade e métodos analíticos. 2009;32:1596–608.
ASSOCIACÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 15764: Biodiesel—Determinacão do teor total de ésteres por cromatografia gasosa; 2015.
Henderson RK, Jiménez-González C, Constable DJC, Alston SR, Inglis GGA, Fisher G, et al. Expanding GSK’s solvent selection guide: embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011. https://doi.org/10.1039/c0gc00918k.
Santos AGD, Araujo AS, Caldeira VPS, Fernandes VJ, Souza LD, Barros AK. Model-free kinetics applied to volatilization of Brazilian sunflower oil, and its respective biodiesel. Thermochim Acta. 2010. https://doi.org/10.1016/j.tca.2010.04.015.
Mothé CG, De Castro BCS, Mothé MG. Characterization by TG/DTG/DSC and FTIR of frying and fish oil residues to obtain biodiesel. J Therm Anal Calorim. 2011. https://doi.org/10.1007/s10973-011-1795-z.
Sousa FP, Luciano MA, Pasa VMD. Thermogravimetry and viscometry for assessing the ester content (FAME and FAEE). Fuel Process Technol. 2013. https://doi.org/10.1016/j.fuproc.2012.09.049.
Vega-Lizama T, Díaz-Ballote L, Hernández-Mézquita E, May-Crespo F, Castro-Borges P, Castillo-Atoche A, et al. Thermogravimetric analysis as a rapid and simple method to determine the degradation degree of soy biodiesel. Fuel. 2015. https://doi.org/10.1016/j.fuel.2015.04.047.
Da Silva JCT, Gondim AD, Galvão LPFC, Da Costa Evangelista JP, Araujo AS, Fernandes VJ. Thermal stability evaluation of biodiesel derived from sunflower oil obtained through heterogeneous catalysis (KNO3/Al2O3) by thermogravimetry. J Therm Anal Calorim. 2015. https://doi.org/10.1007/s10973-014-4145-0.
Gaglieri C, Alarcon RT, de Moura A, Mendes RA, Caires FJ. Is thermogravimetry an efficient alternative to gas chromatography in degree of biodiesel conversion? J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7364-y.
Almazrouei M, Elagroudy S, Janajreh I. Transesterification of waste cooking oil: quality assessment via thermogravimetric analysis. Energy Proced. 2019. https://doi.org/10.1016/j.egypro.2019.01.478.
Ionashiro M. Giolito: fundamentos de termogravimetria e análise térmica diferencial/calorimetria exploratória diferencial. 2nd ed. Giz Editorial: São Paulo; 2014.
Verma P, Sharma MP. Review of process parameters for biodiesel production from different feedstocks. Renew Sustain Energy Ver. 2016. https://doi.org/10.1016/j.rser.2016.04.054.
da Silva JCM, Nicolau CL, Cabral MRP, Costa ER, Stropa JM, Silva CAA, et al. Thermal and oxidative stabilities of binary blends of esters from soybean oil and non-edible oils (Aleurites moluccanus, Terminalia catappa, and Scheelea phalerata). Fuel. 2020. https://doi.org/10.1016/j.fuel.2019.116644.
European Committee for Standardization. EN-14103: Determination of ester and linolenic acid methyl ester contents. 2003; 1–11.
Malz F, Jancke H. Validation of quantitative NMR. J Pharm Biomed Anal. 2005. https://doi.org/10.1016/j.jpba.2005.01.043.
De Medeiros EJL, Do Egypto Queiroga RCR, De Souza AG, Cordeiro AMTM, De Medeiros AN, De Souza DL, et al. Thermal and quality evaluation of vegetable oils used in ruminant feed. J Therm Anal Calorim. 2013; doi:https://doi.org/10.1007/s10973-012-2653-3.
Zhang Q, Saleh ASM, Chen J, Sun P, Shen Q. Monitoring of thermal behavior and decomposition products of soybean oil: an application of synchronous thermal analyzer coupled with fourier transform infrared spectrometry and quadrupole mass spectrometry. J Therm Anal Calorim. 2014. https://doi.org/10.1007/s10973-013-3283-0.
Paul AK, Achar SK, Dasari SR, Borugadda VB, Goud VV. Analysis of thermal, oxidative and cold flow properties of methyl and ethyl esters prepared from soybean and mustard oils. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6424-z.
de Oliveira TF, Dweck J. Liquid phase oxidation quantitative analysis of biodiesel/diesel blends by differential TG and DTA. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7298-4.
Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry, vol 46, 6th ed. New York: 2010; doi:https://doi.org/10.1198/tech.2004.s248.
Chand P, Reddy CV, Venkat JG, Wang T, Grewell D. Thermogravimetric quantification of biodiesel produced via alkali catalyzed transesterification of soybean oil. Energy Fuels. 2009. https://doi.org/10.1021/ef800668u.
Acknowledgements
The authors are grateful for the financial support provided by the Mato Grosso do Sul Foundation for the Promotion of Teaching, Science, and Technology Development (Fundect-MS, Brazil) and the Brazilian Council for Scientific and Technological Development (CNPq). This investigation was partly funded by the Coordination for the Improvement of Higher Education Personnel (Capes, Brazil; finance code 001) and by the Universidade Federal de Mato Grosso do Sul.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cortes, M.R., de Queiroz, J.F., dos Santos, T.M.N. et al. Applying quasi-isothermal thermogravimetry to determine degrees of oil conversion into biodiesel. J Therm Anal Calorim 147, 4397–4402 (2022). https://doi.org/10.1007/s10973-021-10834-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10834-y