Abstract
Yunnan anthracite coal (YN) with high FeS2, Pingshuo coal (PS) with high organic sulfur and Duerping coal (DEP) with 33.52% organic and 64.20% inorganic sulfur were selected to investigate the effects of minerals and Fe3+ on sulfur release behavior during coal pyrolysis under Ar and 3% O2–Ar atmospheres by pyrolysis connected with gas chromatogram (Py-GC) combined with pyrolysis coupled with mass spectrometer (Py-MS). It is found that the sulfur removal ratios and the total amount of sulfur-containing gases release of coal samples under 3% O2–Ar atmosphere are higher than those under Ar atmosphere, indicating that O2 participates in coal pyrolysis process. The effects of minerals on sulfur removal are affected by these coals types. However, effects of Fe3+ on sulfur removal are related to atmospheres. During pyrolysis, Fe3+ is beneficial for all sulfur compounds to decompose at lower temperature under 3% O2–Ar atmosphere, while inhibits sulfur removal under Ar atmosphere. The minerals in coals can absorb sulfur gases. However, Fe3+ can significantly promote COS and SO2 release at lower temperatures under both atmospheres. But Fe3+ inhibits H2S release under Ar atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The utilization of coal in modern society to drive rapid economic development has caused a sharp decline in the reserves of high-quality coal. Thus, the consumption amount of inferior coal would increase. The sulfur content in the inferior coal is high, so some generated sulfur-containing gases can bring a series of environmental problems during coal utilization [1,2,3,4,5,6]. Therefore, in order to reduce environmental pollutions, it is necessary to know the sulfur transformation behavior during coal utilization.
Pyrolysis has been known as a kind of coal clean utilization technology and received more and more attention in improving coal utilization efficiency and controlling environmental pollutions. Therefore, it is important to extensively investigate the sulfur transformation behavior during coal pyrolysis [7,8,9,10,11,12,13,14]. Recently, many scholars have studied the effects of additives on sulfur release and migration during coal pyrolysis and have drawn some important conclusions. Zhang et al. [15] has found that both CaO and Fe2O3 can inhibit the decomposition of sulfate sulfur in raw coal and the release of SO2 from pyrolysis solid combustion. Jia et al. [16] has reported that the addition of CaSO4 can increase the yield of H2S and COS, and a high proportion of CaSO4 can promote the decomposition of organic sulfur in tar and char. Wang et al. [17] considered that tourmaline, Na-bentonite and medical stone can increase the preservation rate of total sulfur and organic sulfur and reduce the preservation rate of sulfate sulfur. Huang et al. [18] investigated that chromium ions promote the decomposition of unstable organic sulfur at low temperature, and the effect of sulfur-fixing agent inhibits the release of H2S at 400–700 °C.
However, most of these studies [19,20,21,22] have only focused on the use of Py-GC or Py-MS to investigate sulfur release and migration in inert atmosphere. Only a few reports are available on the effects of low concentration oxygen atmosphere and ions on sulfur transformation during deashed coal pyrolysis. In this paper, three coal samples with different kinds of sulfur forms and their deashed coals with Fe3+ were selected to study sulfur release and transformation behavior under Ar and 3% O2–Ar atmospheres by Py-MS combined with Py-GC. Only after the detailed sulfur release and its transformation behavior are investigated during pyrolysis of raw coals, deashed coals and deashed coals with Fe3+, the influence of minerals and Fe3+ on sulfur release behavior and sulfur removal can be recognized under different atmospheres. This can provide some theoretical guidance for the clean utilization of inferior high-sulfur coal.
Experimental
Samples
Three kinds of coal samples, Yunnan anthracite coal (YNR) with high FeS2, Pingshuo coal (PSR) with high organic sulfur and Duerping coal (DEPR) with 33.52% organic and 64.20% inorganic sulfur, were selected, crushed by a sealed pulverizer, sieved to a particle size range of 0.154–0.258 mm by a vibrating screen machine and stored in brown jars for further experiment. The minerals in these coals were removed by the HCl-HF-HCl method [23]. The corresponding deashed coals were labeled by YNDA, PSDA and DEPDA, respectively. The proximate and ultimate analyses of coal samples are shown in Table 1. According to the content of volatilization and fixed carbon in different coals, the carbonization degree order of these coals is YNR > DEPR > PSR. Table 2 lists the sulfur form analyses of these coals. The ash composition analyses of these raw coals are shown in Table 3. The proximate analyses were analyzed according to the Chinese standard methodology GB/T 212-2008, and ultimate analyses data were obtained by a Vario EL elemental analyzer. The ash analyses and the sulfur forms analyses followed the Chinese standard methodology GB/T 1574-2007 and GB/T 215-2003, respectively.
Fe3+ was loaded into coal according to the mass fraction of metal by impregnation method. The detailed steps are as follows: 5 g of coal was weighed accurately and then 50 mL of 0.036 mol L−1 of FeCl3 solution was added. Then, the obtained mixture was agitated for 6 h at room temperature, let to stand for 6 h and then put in an oven and dried at 60 °C. The prepared samples were collected and labeled as DEPDA with 2% Fe3+, YNDA with 2% Fe3+, PSDA with 2% Fe3+ and saved in brown bottles in the dark place [24].
Py-GC equipment
About 1 g of sample was placed into a fixed bed reactor and heated from room temperature to 900 °C at a heating rate of 10 °C min−1 in a continuous flow of pure Ar or 3% O2–Ar atmosphere at a flow rate of 0.3 L min−1. H2S, COS and SO2 contents (ppm) were analyzed by gas chromatography with flame photometric detector (GC-FPD) (SP-7800) every 50 °C, off-line. The column and detector temperatures were 80 °C and 250 °C, respectively [9, 11, 14]. Ventilation was performed for 30 min before each experiment to eliminate air interference in the reaction apparatus.
Py-MS experiment
Pyrolysis experiments were carried out in a vertical fixed bed quartz tube reactor (i. d. 30 mm, length 60 cm). About 1 g of sample was pyrolyzed under pure Ar or 3% O2–Ar atmosphere at the temperature range from room temperature to 900 °C at a flow rate of 200 mL min−1 at a heating rate of 10 °C min−1. In the experiment, the gaseous products of H2S, COS and SO2 were measured by an online MS (Hiden QIC-20) [11].
Calculating methods
The detailed calculating methods of char yield (Y), desulfurization ratio (DR) and total sulfur release amount and H2S, COS and SO2 release amount in the gas phase can be seen in the literature [9, 12]. According to those equations, the char yields and desulfurization ratios of these coals are listed in Table 4, and the total sulfur release amount and H2S, COS and SO2 release amount in different atmospheres are shown in Table 5.
Results and discussion
Effects of minerals and Fe3+ on the char yields and desulfurization ratios of coal samples
Table 4 shows the char yields and desulfurization ratios of coal samples during pyrolysis under Ar and 3% O2–Ar atmospheres. Under these two atmospheres, the order of char yields of these coals is deashed coal with 2% Fe3+ < deashed coal < raw coal. This indicates that Fe3+ is beneficial for coal decomposition. Fe3+ can destroy hydrogen bonds in coal and form active complex with the functional groups on coal surface, so the volatile matter of the coal increases, resulting in the decreasing final char yields [25, 26]. The char yields of these raw coals are all higher than those of their corresponding deashed coals, suggesting that the minerals in raw coals can inhibit mass transferring and heat transferring. In addition, the char yields order of raw coals under two atmospheres is YN > DEP > PS. This is consistent with the carbonization degree of these three coals (Table 1). The char yields order of same coal sample is Ar > 3% O2–Ar, indicating that O2 can participate in coal pyrolysis and break more C–C bonds [27].
Under the same atmosphere, the effects of minerals on sulfur removal are different for different coals. For DEP coal, the sulfur removal ratios of its raw coal are lower than those of its deashed coal under Ar and 3% O2–Ar atmospheres. This suggests that minerals in DEP raw coal have no obvious catalytic effect on sulfur decomposition and mainly inhibit the mass transferring and heat transferring and/or absorb sulfur-containing gases. For YN and PS coals, their sulfur removal ratios decrease after deashed treatment. This indicates that the minerals in these two coals can catalyze sulfur decomposition. Under different atmospheres, the effects of minerals on the desulfurization ratio of same coal are similar. The desulfurization ratios of the same samples are all higher under 3% O2–Ar than Ar atmosphere, suggesting that O2 can also participate in sulfur decomposition and break more C–S bonds [27, 28].
Fe3+ obviously inhibits sulfur removal under Ar atmosphere. The most possible reason is that Fe3+ can react with the sulfur-containing gases and form metal sulfide difficultly decomposing under inert atmosphere [25]. Fe3+ promotes sulfur removal under 3% O2–Ar atmosphere, as the formed metal sulfide can react with O2 to increase the desulfurization ratios.
Effects of minerals and Fe3+ on the release amount of sulfur-containing gases during coal pyrolysis
The release amount of various sulfur-containing gases can be quantitatively analyzed by Py-GC. Table 5 shows the sulfur gases and total sulfur releasing amount during pyrolysis under different atmospheres. It can be seen that the sulfur-containing gases release into gas phase mainly in the form of H2S during coal pyrolysis under Ar atmosphere, while mainly SO2 under 3% O2–Ar atmosphere. The total release amount of sulfur-containing gases of same coal under 3% O2–Ar atmosphere is higher than that under Ar atmosphere, indicating that O2 can promote more sulfur-containing compounds to decompose. This is consistent with higher sulfur removal ratios under 3% O2–Ar atmosphere as above discussion.
H2S, COS and SO2 release amount and total sulfur release amount of DEPDA are all lower than those of its raw coal under Ar and 3% O2–Ar atmospheres. This indicates that the minerals in DEPR can promote the sulfur release into gas phase. Except for SO2 release of YNDA under Ar, these sulfur-containing gases release amount and total sulfur releases amount of YNDA are all much higher than those of YNR under Ar and 3% O2–Ar atmospheres. This suggests that alkaline minerals in YNR can obviously absorb these sulfur-containing gases. And the reason of the lower SO2 release amount of YNDA under Ar is that FeSO4 resulted from the oxidation of high pyrite and can be removed during deashed treatment. And it can also be seen that the content of FeSO4 in YNDA decreases significantly in Table 2. For PSDA, these sulfur-containing gases release amount and total sulfur release amount are all much higher than those of PSR under Ar, while lower under 3% O2–Ar atmosphere except for COS release. This suggests the minerals mainly possess the alkaline absorption under Ar atmosphere and promoting decomposition of sulfur under 3% O2–Ar atmosphere.
Except for SO2 release of YNDA with 2% Fe3+, Fe3+ prevents all sulfur-containing gases of these coals from releasing under Ar atmosphere, while Fe3+can promote SO2 release and total sulfur release amount to increase under 3% O2–Ar atmosphere. This is consistent with lower sulfur removal ratios of these deashed coals with 2% Fe3+ under Ar atmosphere and their higher sulfur removal ratios under 3% O2–Ar atmosphere in Table 4.
Effects of minerals and Fe3+ on the release of sulfur-containing gases during coal pyrolysis
Effects of minerals and Fe3+ on H2S release during pyrolysis
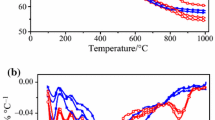
Figure 1 shows the release curves of H2S during pyrolysis of these three raw coals, their deashed coals and deashed coals with 2% Fe3+ under Ar atmosphere. During pyrolysis, pyrite and unstable organic sulfur can decompose and form active elemental sulfur (Sn) or sulfur hydrogen radical (SH·), which can react with H provided by coal structure to form H2S [25, 29, 30]. For DEPR and YNR, only one H2S release peak was detected by Py-MS, and their H2S release peaks should be mainly attributed to the decomposition of pyrite. It is known that most of sulfur is pyrite in YN coal (Table 2) and the organic sulfur in DEP coal are very stable and cannot easily decompose under Ar atmosphere as its highest coal rank among these coals (Table 1). For PSR, a very wide H2S peak was detected by MS. The main H2S peak at 561 °C is mainly generated by the decomposition of unstable organic sulfur, as the coal rank of PS coal is the lowest among these coals. And its tail peak should be resulted from the decomposition of stable organic sulfur. For these three deashed coals, the maximum release peak temperatures of H2S all move to lower temperature and their H2S intensities are all higher than their raw coals. This indicates that the heat transferring and mass transferring effects become stronger and the absorption of alkaline minerals disappears after deashed treatment. Fe3+ makes the maximum release peak temperatures of H2S move toward higher temperatures. Meanwhile, H2S intensity of these deashed coals with 2% Fe3+ is all lower than their own deashed coals, suggesting 2% Fe3+ can hinder H2S release. This is consistent with the H2S release amount that is lower in deashed coals with 2% Fe3+ than that in deashed coals in Table 5.
Figure 2 shows the release profiles of H2S of raw coals, deashed coals and deashed coals with 2% Fe3+ during pyrolysis under 3% O2–Ar atmosphere. No significant H2S release was detected by MS during pyrolysis of all these samples, as the MS fragments of H2S are similar to those of O2.
Effects of minerals and Fe3+ on COS release during pyrolysis
COS release profiles detected by MS are shown in Fig. 3 during pyrolysis of these raw coals, deashed coals and deashed coals with 2% Fe3+ under Ar atmosphere. Except for DEPDA with 2% Fe3+, only one obvious COS release peak was detected by MS during pyrolysis for other coals. For YNR and DEPR, COS release peaks should be mainly attributed to the decomposition of pyrite. And for PSR, the lower part of the wide COS peak should be attributed to unstable organic decomposition and the higher part is related to stable organic sulfur decomposition. For DEPDA with 2% Fe3+, the first COS release peak comes from the decomposition of unstable organic sulfur, and the second peak is related to pyrite decomposition. Similar to H2S release, the maximum COS release peak temperatures of these three deashed coals all move toward the lower temperatures compared with their raw coals. Similarly, their COS intensities are all stronger than their raw coals. This suggests minerals in these three raw coals have obvious absorption effects under Ar atmosphere. Fe3+ can promote the maximum peak temperatures of COS release to decrease, indicating that Fe3+ was beneficial for COS to release at lower temperatures [25], especially for PSDA and DEPR coals with higher organic sulfur. This suggests that Fe3+ can promote the decomposition of organic sulfur-containing compounds at lower temperatures under Ar atmosphere. But the COS intensity of each deashed coal with Fe3+ is lower than their deashed coals, which is inconsistent with the COS release amount in Table 5.
As shown in Fig. 4, the maximum COS release peak of DEPR should be related to the decomposition of stable organic sulfur and FeS, and the shoulder peak before main peak is related to the decomposition of unstable organic sulfur and pyrite. And the main COS release peak of YN coal should also be related to FeS decomposition and the shoulder peak before the main peak is from pyrite decomposition under 3% O2–Ar atmosphere. Similar to H2S release of PSR, the lower part of its wide COS peak should be attributed to unstable organic decomposition and the higher part is related to stable organic sulfur decomposition. The minerals in DEPR inhibit COS release as the alkaline minerals can absorb sulfur-containing gases. Thus, COS release peak temperature of DEPDA decreases much after minerals were removed. However, the minerals in YNR and PSR can promote COS release at lower temperatures under 3% O2–Ar atmosphere, which is obviously different from their trends under Ar atmosphere. This indicates that the effects of minerals on COS release are different under different atmospheres. Fe3+ can also promote COS release at lower temperatures, which is consistent with the trend under Ar atmosphere. After 750 °C, COS release intensity of these three deashed coals with 2% Fe3+ increases with the increase in temperature. This indicates that Fe3+ can promote the decomposition of more stable organic sulfur and more COS release into the gas phase. This is consistent with the COS release amount of DEPDA and YNDA with 2% Fe3+ that is higher than that of their deashed coals (seen in Table 5).
Effects of minerals and Fe3+ on SO2 release during pyrolysis
Figure 5 shows the SO2 release profiles detected by MS during pyrolysis of theses samples under Ar atmosphere. Except for PSR, two SO2 release peaks of DEPR and YNR were detected by MS under Ar atmosphere. The first unobvious small peak of YNR should be mainly attributed to the decomposition of sulfate since pyrite is about 97% and is easily oxidized to FeSO4. The first peak of DEPR should be mainly related to the decomposition peak of unstable organic sulfur and sulfate. And their second peak is mainly from the decomposition of pyrite. For PSR, the wide SO2 release peak should be mainly related to the decomposition of unstable organic sulfur and the maximum peak temperature is the lowest, as the unstable organic sulfur content of PS coal is high because of its low carbonization degree. The minerals of these three raw coals can absorb SO2 under Ar atmosphere and hinder heat transferring and mass transferring, so the SO2 release peak temperatures of these three deashed coals all moved to the lower temperatures. As shown in Fig. 5, Fe3+ can promote unstable sulfur and pyrite to decompose at lower temperatures.
As shown in Fig. 6, for all these samples, a very wide release peak of SO2 was detected during pyrolysis under 3% O2–Ar atmosphere. The maximum SO2 release peak temperatures of all these deashed coals all decrease. This is very similar to their SO2 release under Ar atmosphere. This further testifies that the minerals of these three raw coals can absorb SO2 under Ar atmosphere and hinder heat transferring and mass transferring. This is consistent with higher SO2 release amount of these deashed coals (Table 5). Interestingly, unlike other coals, SO2 release intensity of YNR and YNDA remains steady after 510 °C and 473 °C, respectively. It is known that pyrite is very high in YNR (Table 2) and pyrite can transfer FeS and stable organic sulfur. As FeS and stable organic can be oxidized to SO2 at higher temperatures under 3% O2–Ar atmosphere, SO2 release intensity remains steady for YNR and YNDA. Fe3+ can make the starting release temperature and the maximum release temperature of SO2 all move toward the lower temperatures. This suggests that Fe3+ is helpful for unstable organic sulfur, pyrite and stable organic sulfur to decompose at lower temperatures under 3% O2–Ar atmosphere. This can be further proved by higher sulfur removal ratios (Table 4) and higher SO2 release amount of these deashed coals with 2% Fe3 + (Table 5).
Conclusions
In this study, Py-GC combined with Py-MS were used to study the effects of minerals in coals and Fe3+ on sulfur gases release behavior under Ar and 3% O2—Ar atmospheres, and the following conclusions can be drawn:
Under the same atmosphere, the effects of minerals on sulfur removal are related to the coals types. Under different atmospheres, effects of minerals on the desulfurization ratios are similar to the same coal in different atmospheres. Fe3+ obviously inhibits sulfur removal under Ar atmosphere, while promotes sulfur removal under 3% O2–Ar atmosphere.
For sulfur-containing gases, Fe3+ inhibits H2S release at lower temperatures under Ar atmosphere, while promotes COS and SO2 release at lower temperatures under both atmospheres. The minerals inhibit H2S and SO2 release at low temperatures under both atmospheres.
References
Zhang Q, Nakatani J, Shan Y, Moriguchi Y. Inter-regional spillover of China's sulfur dioxide (SO2) pollution across the supply chains. J Clean Prod. 2019;207:418–31.
Ling Z, Huang T, Li J, Zhou S, Lian L, Wang J, Zhao Y, Mao X, Gao H, Ma J. Sulfur dioxide pollution and energy justice in Northwestern China embodied in West-East Energy Transmission of China. Appl Energy. 2019;238:547–60.
Gao J, Zhang Y, Meng D, Jiao T, Qin X, Bai G, Liang P. Effect of ash and dolomite on the migration of sulfur from coal pyrolysis volaties. J Anal Appl Pyrol. 2019;140:349–54.
Kanaroglou P, Adams M, De Luca P, Corr D, Sohel N. Estimation of sulfur dioxide air pollution concentrations with a spatial autoregressive model. Atmos Environ. 2013;79:421–7.
Gao X, Zheng C. Air pollution control for a green future. J Zhejiang Univ Sci A. 2018;19:1–4.
Zhou Y, Hao F, Meng W, Fu J. Scenario analysis of energy-based low-carbon development in China. J Environ Sci. 2014;26:1631–40.
Liu F, Li B, Li W, Bai Z, Yperman J. Py-MS study of sulfur behavior during pyrolysis of high-sulfur coals under different atmospheres. Fuel Process Technol. 2010;91:1486–90.
Wang M, Liu L, Wang J, Chang L, Wang H, Hu Y. Sulfur K-edge XANES study of sulfur transformation during pyrolysis of four coals with different ranks. Fuel Process Technol. 2015;131:262–9.
Wang X, Guo H, Liu F, Hu R, Wang M. Effects of CO2 on sulfur removal and its release behavior during coal pyrolysis. Fuel. 2016;165:484–9.
Qi T, Li W, Chen H, Li B. Desulfurization of coal through pyrolysis in a fluidized-bed reactor under nitrogen and 0.6% O2–N2 atmosphere. Fuel. 2004;83:705–12.
Guo H, Wang X, Liu F, Wang M, Zhang H, Hu R, Hu Y. Sulfur release and its transformation behavior of sulfur-containing model compounds during pyrolysis under CO2 atmosphere. Fuel. 2017;206:716–23.
Xu W, Kumagai M. Sulfur transformation during rapid hydropyrolysis of coal under high pressure by using a continuous free fall pyrolyzer. Fuel. 2003;82:245–54.
Liu Q, Hu H, Zhu S, Zhou Q, Li W, Wei X, Xie K. Desulfurization of coal by pyrolysis and hydropyrolysis with addition of KOH/NaOH. Energy Fuels. 2005;19:1673–8.
Yang N, Guo H, Liu F, Zhang H, Hu Y, Hu R. Effects of atmospheres on sulfur release and its transformation behavior during coal thermolysis. Fuel. 2018;215:446–53.
Zhang Y, Liang P, Jiao T, Wu J, Zhang H. Effect of foreign minerals on sulfur transformation in the step conversion of coal pyrolysis and combustion. J Anal Appl Pyrol. 2017;127:240–5.
Jia X, Wang Q, Han L, Cheng L, Fang M, Luo Z, Cen K. Sulfur transformation during the pyrolysis of coal with the addition of CaSO4 in a fixed-bed reactor. J Anal Appl Pyrol. 2017;124:319–26.
Wang B, Zhao S, Huang Y, Zhang J. Effect of some natural minerals on transformation behavior of sulfur during pyrolysis of coal and biomass. J Anal Appl Pyrol. 2014;105:284–94.
Huang J, Bai Z, Guo Z, Li W, Bai J. Effects of chromium ion on sulfur removal during pyrolysis and hydropyrolysis of coal. J Anal Appl Pyrol. 2012;97:143–8.
Shen Y, Wang M, Hu Y, Kong J, Wang J, Chang L, Bao W. Transformation and regulation of sulfur during pyrolysis of coal blend with high organic-sulfur fat coal. Fuel. 2019;249:427–33.
Guo H, Fu Q, Zhang L, Liu F, Hu Y, Zhang H, Hu R. Sulfur K-edge XAS study of sulfur transformation behavior during pyrolysis and co-pyrolysis of biomass and coals under different atmospheres. Fuel. 2018;234:1322–7.
Wang M, Jia T, Wang J, Hu Y, Liu F, Wang H, Chang L. Changes of sulfur forms in coal after tetrachloroethylene extraction and theirs transformations during pyrolysis. Fuel. 2016;186:726–33.
Jia X, Wang Q, Cen K, Cheng L. Sulfur transformation during the pyrolysis of coal mixed with coal ash in a fixed bed reactor. Fuel. 2016;177:260–7.
Zhang J, Wang M, Chen W, Fu C, Ren X, Chang L. Effects of step acid treatment process on the structure and pyrolysis characteristics of Ximeng brown coal: formation of gaseous products. J Fuel Chem Technol. 2013;41:1160–5.
Chang L, Qin Z, Wang M, Zhang Y. Effects of FeCl3 addition on transformation of organic sulfur during the pyrolysis upgrading of Ximeng brown coal. J Taiyuan Univ Technol. 2012;43:406–10.
Huang J, Bai Z, Guo Z, Li W, Bai J. Effects of impregnated iron and chrome on gas evolution of Lingshi coking coal during pyrolysis. J China Univ Min Technol. 2012;41:613–9.
Miura K, Mae K, Sakurada K, Hashimoto K. Flash pyrolysis of coal following thermal pretreatment at low temperature. Energy Fuels. 1992;6:16–211.
Guo H, Zhao L, Ma Q, Liu F. Sulfur removal and transformation of Huozhou coal during pyrolysis under oxidative atmosphere. Coal Convers. 2007;2:10–3.
Liu F, Li W, Chen H, Li B. Uneven distribution of sulfurs and their transformation during coal pyrolysis. Fuel. 2007;86:360–6.
Lv D, Xu M, Yao H, Liu X, Jiang W, Cao H, Zhan Z. Effects of indigenous and added minerals on transformation of organic and inorganic sulfur in density separated coal fractions during CO2-pyrolysis. Energy Fuels. 2010;24:123–30.
Yan J, Yang J, Liu Z. SH radical: the key intermediate in sulfur transformation during thermal processing of coal. Environ Sci Technol. 2005;39:5043–51.
Acknowledgements
This study was financially supported by the Projects of Natural Science Foundation of China (No. 21865018) and the Project Natural Science Foundation of Inner Mongolia (No. 2018MS02005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, Y., Wang, J., Guo, H. et al. Effects of minerals and Fe3+ on sulfur removal and its release behavior during coal pyrolysis under different atmospheres. J Therm Anal Calorim 142, 2319–2326 (2020). https://doi.org/10.1007/s10973-020-09551-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09551-9