Abstract
The aim of this study was to investigate the effect of calcium hypophosphite (CaHP) and magnesium hypophosphite (MgHP) on the thermal stability and fire retardant properties of cotton fabrics. The effects of water-soluble nanoparticles, namely Na-montmorillonite and octaammonium polyhedral oligomeric silsesquioxane (OA-POSS), in the presence of CaHP were also investigated. The characterizations of flame-retardant-treated cotton fabrics were performed using thermogravimetric analysis, limiting oxygen index (LOI) and mass loss calorimeter studies. The residues remained after mass loss calorimeter test were characterized by conducting attenuated total reflectance Fourier-transform infrared spectroscopy and scanning electron microscopy with a wavelength-dispersive X-ray spectrometer. The results showed that the fire performance of cotton fabric increased as the treated amount of both hypophosphite compounds increased. CaHP showed better fire performance than MgHP. The fire retardant performance of CaHP was further improved when used with nanoparticles. The highest LOI value of 24.2 and the lowest peak heat release rate (PHRR) of 43 ± 3 kW m−2 were achieved with the use of CaHP and OA-POSS at a ratio of 19:1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton, which is the most widely used natural textile fiber, finds use in many applications in the form of woven, knitted or nonwoven owing to its good comfort, permeability and biodegradability properties. However, the flammable character of cotton restricts its use in many fields. Thus, nondurable, semi-durable or durable flame-retardant finishing is applied depending upon the application area [1, 2]. In recent years, different phosphorus-based compounds with various oxidation states are widely used for providing flame retardant property to cotton-based textile materials due to their remarkable fire retardant performance with environmentally friendly characters [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18].
Different metal salts of hypophosphites, especially aluminum hypophosphite, are widely used as flame-retardant additive in engineering plastics [19, 20]. Magnesium, calcium and sodium hypophosphites, which are water-soluble compounds, also find use in flame retardant applications [20,21,22]. It is thought that they can be used as a nondurable flame-retardant finishing agent for cotton fabric. In the literature, the flame-retardant effect of sodium hypophosphite as a catalyst is investigated in cotton fabric [23,24,25,26,27]. As our best knowledge, this is the first study that investigates the effect of calcium hypophosphite (CaHP) and magnesium hypophosphite (MgHP) on the thermal stability and flammability properties of cotton fabrics.
In the second part of the study, the more effective hypophosphite compound (CaHP) is determined and its performance is tried to be improved using nanoparticles, namely Na-montmorillonite and octaammonium polyhedral oligomeric silsesquioxane (OA-POSS). POSS and clay nanoparticles are used as synergistic additives with various phosphorus compounds [28, 29]. No study that investigates the interaction among CaHP, Na-montmorillonite and OA-POSS nanoparticles in any polymeric material is found in the literature. These aforementioned points are the novel part of the current study. Thermal stability and flammability properties of the cotton fabrics are investigated by thermogravimetric analysis (TGA), limiting oxygen index (LOI) and mass loss calorimeter studies. The char residues remained after mass loss calorimeter test are investigated by conducting attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR) and scanning electron microscopy with wavelength-dispersive X-ray spectrometer (SEM-WDX).
Experimental
Materials
Scoured knitted 100% cotton fabric was obtained from KARSU TEKSTIL (Kayseri, Turkey). Calcium hypophosphite (Ca(H2PO2)2) and magnesium hypophosphite ((Mg(H2PO2)2·6 H2O)) were purchased from Wuhan Ruiji Chemical Co., Ltd. OA-POSS was purchased from Hybrid Plastics. Na-montmorillonite was purchased from Sigma-Aldrich.
Production of flame-retardant fabrics
CaHP and MgHP aqueous solutions of 5, 10 and 20 mass% were prepared for flame retardant applications. Before the flame-retardant treatment, cotton fabrics were dried at 60 °C for 24 h. Cotton fabrics were treated with the aqueous solution of hypophosphites at 60 °C for 30 min and padded through the laboratory padder (Termal, Turkey) to control wet pickup of 100% on the fabrics. The treated fabrics were dried at 90 °C for 8 min. For synergistic effect studies, 20 mass% aqueous solutions of CaHP, Na-montmorillonite or OA-POSS were prepared. The solutions of OA-POSS/CaHP and Na-montmorillonite/CaHP were prepared at the mass ratio of 1, 5 and 10%. The applied fire-retardant additive on cotton fabrics was calculated by mass difference before and after treatment. The resulting fabrics contained different amounts of flame-retardant additives due to their affinity difference. The amounts of applied materials are given in Table 1. For sample coding, the abbreviations C, CaHP, MgHP, POSS and clay are used for cotton fabric, calcium hypophosphite, magnesium hypophosphite, OA-POSS nanoparticle and Na-montmorillonite, respectively. The sample coded as C/20 CaHP represents the flame-retardant-finished cotton fabric with 20 mass% CaHP-containing solution. The sample coded as C/90CaHP/10 POSS refers to the cotton fabric treated with 20 mass% solution containing CaHP and OA-POSS nanoparticle at a ratio of 9:1.
Characterization methods
LOI values of the fabrics were measured using Fire Testing Technology Limiting Oxygen Index Analyzer instrument, according to the standard oxygen index test ASTM D2863. TGA was performed on PerkinElmer Diamond TG/DTA at a heating rate of 10 °C min−1 up to 700 °C under nitrogen flow of 50 mL min−1. The mass loss calorimeter test was carried out following the procedures in ISO 13927 using mass loss cone with thermopile attachment (Fire Testing Technology, UK). Square specimens (10 × 10 cm2) were irradiated at a heat flux of 35 kW m−2, corresponding to a mild fire scenario. The test was performed three times for each sample. Residue remained after mass loss calorimeter test was analyzed using SEM-WDX (FEI Quanta 400F) and ATR-FTIR (Bruker Optics IFS 66/S series FTIR spectrometer) at an optical resolution of 4 cm−1 with 32 scans. All sample surfaces were coated with a thin layer of gold with a sputter coater to provide the conductivity.
Results and discussion
Thermal gravimetric analysis
TGA analyses are carried out on flame retardants, pure and flame-retardant-treated cotton fabrics under nitrogen atmosphere. The TGA and DTGA curves of the flame-retardant additives are shown in Fig. 1. The decomposition routes of CaHP and MgHP are shown in Fig. 2. The CaHP degrades mainly in two steps at 417 and 650 °C by leaving 87.4% inorganic residue. A shoulder is seen at 383 °C. In the first step, CaHP decomposes into Ca2(HPO4)2 and PH3. In the second step, Ca2(HPO4)2 decomposes into calcium pyrophosphate and water [22]. MgHP degrades mainly in three steps at 86, 393 and 607 °C. A shoulder is also seen at 375 °C. In the first step, crystal water removes from the structure. In the second step, Mg(H2PO2)2 decomposes into Mg2(HPO4)2 and PH3. In the last step, Mg2(HPO4)2 decomposes into magnesium pyrophosphate and water and leaves 58% residue. Na-montmorillonite decomposes in two steps at 94 and 638 °C and leaves 88% residue. The first step results from the evaporation of absorbed water. The second step stems from the dehydroxylation of the clay [30, 31]. OA-POSS degrades in two steps by leaving 48% residue. The first step stemming from the loss of alkyl ammonium group occurs between 336 and 425 °C with the maximum mass loss temperature at 412 °C. The second step arising from the degradation of Si–O–Si cage structure just starts after the first degradation step and finished at about 600 °C [32].
TGA and DTGA curves of CaHP- and MgHP-treated fabric samples are shown in Fig. 3. The TGA and DTGA curves of fabric samples produced for synergistic effect studies are shown in Fig. 4. The related data are given in Table 2. All fabric samples have mass loss below 100 °C due to the evaporation of the absorbed water. Pure cotton fabric degrades mainly in a single step between 310 and 380 °C with maximum mass loss temperature at 367 °C by leaving 10.2% carbon-based char residue. Cotton fabric mainly degrades by two competitive pathways of depolymerization and dehydration. Depolymerization occurs via the scission of acetal bonds between the glycosidic units, and finally flammable compound, namely levoglucosan, is formed via intramolecular rearrangement. Dehydration reactions cause char formation via transglycosylation reactions [33,34,35,36]. With the treatment of CaHP and MgHP, the initial thermal stability (T5%) and the maximum degradation temperature (Tmax) of cotton fabric are reduced. The char yield of cotton fabric increases as the applied amount of hypophosphite compound increases. It is thought that the thermal stability reduction and the char yield increase arise from the presence of phosphinic acid and hypophosphorous acid which are the decomposition products of CaHP and MgHP [20, 36]. These acidic compounds phosphorylate the primary hydroxyl group of cellulose via esterification reaction which favors cellulose decomposition toward dehydration [37]. Similar trend is observed in the presence of phosphonic acid derivatives in the literature [12]. Accordingly, the levoglucosan formation reduces and the char yield increases. The other reason for char yield increase is the formation of calcium- and magnesium-pyrophosphates-based inorganic residue in the condensed phase.
When CaHP and clay are used together, no meaningful change is observed in T5% values. However, the shoulder seen at about 380 °C becomes distinct when the applied amount of clay reaches 10 mass% of CaHP. It is thought that Bronsted and Lewis acid sites present on the clay lattice make catalytic effect during the decomposition of CaHP [28]. The char yields of clay-containing samples are lower than that of pure CaHP-containing one. When CaHP and OA-POSS are used together, the initial thermal stability is further reduced with respect to C/20 CaHP, owing to the lower thermal stability of OA-POSS nanoparticle. On contrary to other samples, OA-POSS-treated ones degrade in two steps. The first step arises from the degradation of OA-POSS. The second step arises from the degradation of cotton fabric and CaHP. As in the case of Na-montmorillonite, OA-POSS-containing samples have lower char yield than that of C/20 CaHP-containing ones.
Mass loss calorimeter studies
Mass loss calorimeter investigations are performed on all fabric samples for three times. The representative heat release rate (HRR) curves are shown in Fig. 5, and the related data are given in Table 3. The fire performances of fabric samples are evaluated from the peak heat release rate (pHRR) and total heat evolved (THE). The reductions in these data normally indicate improved fire performance.
The neat cotton fabric is ignited in 9 s. With the flame-retardant treatment regardless of the type, TTI value increases. The decrease in levoglucosan formation is the main reason for the increase in TTI. The highest TTI value is observed in C/20 MgHP sample. As stated in the TGA section, MgHP liberates six moles of crystal water at about 90 °C. It is thought that the water vapor dilutes the combustible gases in the gas phase before the ignition. Thus, the TTI value increases.
The neat cotton fabric burns with pHRR and THE values of 101 kW m−2 and 1.28 MJ m−2. It leaves 2.7% char residue. pHRR and THE values reduce, and the char formation increases steadily as the applied amount of hypophosphite compounds increases. pHRR value reduces at about 37 and 31% with 20 mass% CaHP and MgHP treatments, respectively. THE value reduces at about 30 and 38% with 20 mass% CaHP and MgHP treatments, respectively. The proposed flame-retardant actions of the additives are shown in Fig. 6. As shown in Fig. 6, hypophosphite compounds form phosphine gas and water during the decomposition. Water and phosphine gas make a diluting effect in the gas phase. PO2 and PO radicals which are the decomposition products of phosphine gas make scavenger effect in the gas phase [20, 38]. The phosphine gas also reacts with the oxygen and forms phosphoric acid. The formed phosphoric acid either reacts with cellulose or polymerizes to form polyphosphoric acid. The esterification reaction between phosphoric acid and primary alcohol group of cellulose (C6) favors the dehydration rather than depolymerization. Accordingly, the levoglucosan formation reduces and the char formation increases. The formation of cross-linked char structure via the esterification reaction and the formation of calcium and magnesium pyrophosphate compounds also cause char yield increase. These aforementioned points are the possible reasons for reduced pHRR and THE values. The characterization methods performed on the char residues also support the proposed mechanisms.
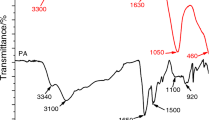
To examine the changes in the surface morphology of cotton fabric after flame-retardant treatment, SEM analysis is carried out. SEM photographs of selected char residues are shown in Fig. 7. The neat cotton fabric has a longitudinal fibrous structure with curled striations. With the flame-retardant treatment, irregularly shaped particles are seen on the fabric surface owing to the formation of phosphate-based phosphorus compounds. ATR-FTIR graphs of the selected char residues are shown in Fig. 8. The char of cotton fabric has characteristics peaks seen at 2920 and 1420 cm−1 arising from stretching and bending vibrations of C–H group. The broad peak seen at 1050 cm−1 stems from the stretching vibration of C–O–C group. The peak at 1710 cm−1 resulting from the stretching vibrations of C=O group shows the formation of ketones and aldehydes via dehydration reaction. The peak at 1580 cm−1 arises from the stretching vibrations of C=C groups in the aromatic char structure [39]. With the treatment of the hypophosphite compounds, the broad peak seen at 1050 cm−1 is split due to the formation of various phosphorus compounds. The peaks seen at 1100 and 880 cm−1 arise from the symmetric and asymmetric stretching vibrations of P–O–P band, suggesting the formation of calcium and magnesium pyrophosphate compounds. The peak seen at 1020 cm−1 results from the stretching vibration of P–O–C group in the phosphate–carbon complexes formed from the esterification reaction between phosphoric acid and cotton. The peak seen at 1280 cm−1 stems from the stretching vibration of P=O group in polyphosphoric acid and cross-linked cellulose phosphate species. The peak seen at 550 cm−1 arises from the Ca–O and Mg–O bond stretching [22, 40]. With the treatment of Na-montmorillonite and OA-POSS, no additional peaks are seen. The Si–O–Si group has stretching and bending vibrations at 1060 and 820 cm−1 which are masked with the peaks of phosphorus compounds [31, 41]. In order to show the presence of Si atom onto the burning surface, SEM-WDX analyses are performed. SEM-WDX results are shown in Fig. 9 and Table 4. The results show that the char of neat cotton fabric is mainly composed of carbon and oxygen. With the treatment of hypophosphite compounds, the content of phosphorus and oxygen of the char increases and carbon content decreases. With the treatment of Na-montmorillonite and OA-POSS, Si atom is seen in the char structure at about 1%.
When Na-montmorillonite and CaHP are used together at the ratios of 1:99 and 10:90, pHRR increases and THE values do not change. The reductions in pHRR and THE values are observed at the ratio of 5:95. It is thought that clay is not sufficient to form a continuous protective layer at low concentration. At higher concentrations, the flame retarding effect of clay cannot compensate for the reduction in the amount of active flame retardant. At optimum concentration, the platelike structure of the clay makes a barrier effect on the burning surface and reduces the heat mass transfer between condensed and gas phases.
When OA-POSS and CaHP are used together, pHRR and THE values reduce at the ratios of 1:99 and 5:95. Further application of OA-POSS causes a slight increase in pHRR owing to the reduction in the amount of active flame retardant. The lowest pHRR and THE values are observed when OA-POSS and CaHP are used together at a ratio of 5:95. The pHRR reduces at about 57%, and THE reduces at about 30% with respect to neat cotton fabric. It is thought that OA-POSS has a dual effect on the fire performance of CaHP. OA-POSS decomposes to form ammonia which makes dilution effect in the gas phase and forms Si–O–Si-based netlike structure which acts as a physical barrier between condensed and gas phases on the burning surface.
Flammability properties
The flammability properties of cotton fabrics are analyzed via LOI test. LOI is a reproducible and quantitative analysis to examine the flammability properties of the polymeric materials. The LOI values of the samples are given in Table 1. The LOI value of a neat cotton sample is 18.2. LOI value increases steadily as the applied amount of hypophosphite compound increases. The higher LOI values with CaHP treatments are observed with respect to MgHP-treated ones. Higher affinity of CaHP causes the higher applied amount of CaHP with respect to MgHP under the same finishing conditions. Accordingly, CaHP shows more fire retardant performance with respect to MgHP. When Na-montmorillonite and CaHP are used together at the ratios of 1:19 and 1:9, the highest LOI value of 23.8 is achieved. When OA-POSS and CaHP are used together at a ratio of 1:19, the highest LOI value of 24.2 is obtained. The increase in LOI values and the reductions in pHRR and THE values clearly show the adjuvant effect of nanoparticles when used with CaHP.
Conclusions
In this study, the possibility of using two hypophosphite compounds, CaHP and MgHP, as flame-retardant agents for cotton fabrics is assessed. The flame retardant performance of CaHP is tried to be improved using water-soluble nanoparticles, namely Na-montmorillonite and OA-POSS. According to TGA results, CaHP and MgHP treatments cause the reduction in T5% and Tmax values and cause an increase in the char yield as the applied amount of hypophosphite compounds increases. According to the mass loss calorimeter results, TTI value of neat cotton fabric increases with the flame-retardant treatment regardless of the type. pHRR and THE values reduce, and the char formation increases steadily as the applied amount of hypophosphite compounds increases. When CaHP and nanoparticles are used together, the fire performance of CaHP is further improved with higher LOI and lower pHRR and THE values. The adjuvant effect of nanoparticles is observed at different concentrations depending upon the type.
References
Weil ED, Levchik SV. Practical applications, flame retardants for plastics and textiles. Munich: Hanser Publisher; 2009. p. 978–83.
Bourbigot S, Duquesne S, Wilkie CA, Morgan AB. Fire retardancy of polymeric materials. Boca Raton: CRC Press; 2010.
Chen Z, Dong C, Li Q, Bai Y, Lu Z. Preparation of linear piperazine/phosphorous/polysiloxane copolymer and its application on cotton fabrics. J Therm Anal Calorim. 2017;130(3):1997–2005.
Dong C, Lu Z, Wang P, Zhu P, Li X, Sui S, Zhang L, Liu J. Flammability and thermal properties of cotton fabrics modified with a novel flame retardant containing triazine and phosphorus components. Text Res J. 2017;87(11):1367–76.
Feng Y, Zhou Y, Li D, He S, Zhang F, Zhang G. A plant-based reactive ammonium phytate for use as a flame-retardant for cotton fabric. Carbohyd Polym. 2017;175:636–44.
Jia Y, Hu Y, Zheng D, Zhang G, Zhang F, Liang Y. Synthesis and evaluation of an efficient, durable, and environmentally friendly flame retardant for cotton. Cellulose. 2017;24(2):1159–70.
Jiang D, Sun C, Zhou Y, Wang H, Yan X, He Q, Guo J, Guo Z. Enhanced flame retardancy of cotton fabrics with a novel intumescent flame-retardant finishing system. Fibers Polym. 2015;16(2):388–96.
Liu X, Zhang Q, Cheng B, Ren Y, Zhang Y, Ding C. Durable flame retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose. 2018;25(1):799–811.
Liu Z, Xu M, Wang Q, Li B. A novel durable flame retardant cotton fabric produced by surface chemical grafting of phosphorus-and nitrogen-containing compounds. Cellulose. 2017;24(9):4069–81.
Rosace G, Colleoni C, Trovato V, Iacono G, Malucelli G. Vinylphosphonic acid/methacrylamide system as a durable intumescent flame retardant for cotton fabric. Cellulose. 2017;24(7):3095–108.
Shariatinia Z, Javeri N, Shekarriz S. Flame retardant cotton fibers produced using novel synthesized halogen-free phosphoramide nanoparticles. Carbohyd Polym. 2015;118:183–98.
Wang S, Sui X, Li Y, Li J, Xu H, Zhong Y, Zhang L, Mao Z. Durable flame retardant finishing of cotton fabrics with organosilicon functionalized cyclotriphosphazene. Polym Degrad Stab. 2016;128:22–8.
Xu L, Wang W, Yu D. Preparation of a reactive flame retardant and its finishing on cotton fabrics based on click chemistry. RSC Adv. 2017;7:2044–50.
Zhao B, Liu YT, Zhang CY, Liu DY, Li F, Liu YQ. A novel phosphoramidate and its application on cotton fabrics: synthesis, flammability and thermal degradation. J Anal Appl Pyrol. 2017;125:109–16.
Zheng D, Zhou J, Wang Y, Zhang F, Zhang G. A reactive flame retardant ammonium salt of diethylenetriaminepenta (methylene-phosphonic acid) for enhancing flame retardancy of cotton fabrics. Cellulose. 2018;25(1):787–97.
Zheng D, Zhou J, Zhong L, Zhang F, Zhang G. A novel durable and high-phosphorous-containing flame retardant for cotton fabrics. Cellulose. 2016;23(3):2211–20.
Zhou L, Liang Z, Li R, Huang D, Ren X. Flame-retardant treatment of cotton fabric with organophosphorus derivative containing nitrogen and silicon. J Therm Anal Calorim. 2017;128(2):653–60.
Zope IS, Foo S, Seah DGJ, Akunuri AT, Dasari A. Development and evaluation of a water-based flame retardant spray coating for cotton fabrics. ACS Appl Mater Interfaces. 2017;9:40782–91.
Wu N, Li X. Flame retardancy and synergistic flame retardant mechanisms of acrylonitrile-butadiene-styrene composites based on aluminum hypophosphite. Polym Degrad Stab. 2014;105:265–76.
Li Q, Li B, Zhang S, Lin M. Investigation on effects of aluminum and magnesium hypophosphites on flame retardancy and thermal degradation of polyamide 6. J Appl Polym Sci. 2012;125:1782–9.
Jian RK, Chen L, Zhao B, Yan YW, Li XF, Wang YZ. Acrylonitrile–butadiene–styrene terpolymer with metal hypophosphites: flame retardance and mechanism research. Ind Eng Chem Res. 2014;53:2299–307.
Tang G, Huang X, Ding H, Wang X, Jiang S, Zhou K, Wang B, Yang W, Hu Y. Combustion properties and thermal degradation behaviors of biobased polylactide composites filled with calcium hypophosphite. RSC Adv. 2014;4:8985–93.
Cheng X, Yang CQ. Flame retardant finishing of cotton fleece fabric: part V. Phosphorus-containing maleic acid oligomers. Fire Mater. 2009;33:365–75.
Lessan F, Montazer M, Moghadam MB. A novel durable flame-retardant cotton fabric using sodium hypophosphite, nano TiO2 and maleic acid. Thermochim Acta. 2011;520:48–54.
Wu X, Yang CQ. Flame retardant finishing of cotton fleece fabric: part III—the combination of maleic acid and sodium hypophosphite. J Fire Sci. 2008;26:351–68.
Yang CQ, Wu W. Combination of a hydroxy-functional organophosphorus oligomer and a multifunctional carboxylic acid as a flame retardant finishing system for cotton: part II. Formation of calcium salt during laundering. Fire Mater. 2003;27:239–51.
Yang CQ, He Q, Voncina B. Cross-linking cotton cellulose by the combination of maleic acid and sodium hypophosphite. 2. Fabric fire performance. Ind Eng Chem Res. 2011;50:5889–97.
Kiliaris P, Papaspyrides CD. Polymer/layered silicate (clay) nanocomposites: an overview of flame retardancy. Prog Polym Sci. 2010;35:902–58.
Laoutid F, Bonnaud L, Alexandre M, Lopez-Cuesta J-M, Dubois Ph. New prospects in flame retardant polymer materials: from fundamentals to nanocomposites. Mater Sci Eng R. 2009;63:100–25.
He H, Tao Q, Zhu J, Yuan P, Shem W, Yang S. Silylation of clay mineral surfaces. Appl Clay Sci. 2013;71:15–20.
Romanzini D, Piroli V, Frache A, Zattera AJ, Amico SC. Sodium montmorillonite modified with methacryloxy and vinylsilanes: influence of silylation on them morphology of clay/unsaturated polyester nanocomposites. Appl Clay Sci. 2015;114:550–7.
Turgut G, Dogan M, Tayfun U, Ozkoc G. The effects of POSS particles on the flame retardancy of intumescent polypropylene composites and the structure-property relationship. Polym Degrad Stab. 2018;149:96–111.
Fang F, Chen X, Zhang X, Cheng C, Xiao D, Meng Y, Ding X, Zhang H, Tian X. Environmentally friendly assembly multilayer coating for flame retardant and antimicrobial cotton fabric. Prog Org Coat. 2016;90:258–66.
Liu Y, Pan YT, Wang X, Acuna P, Zhu P, Wagenknecht U, Heinrich G, Zhang XQ, Wang R, Wang DY. Effect of phosphorus containing inorganic organic hybrid coating on the flammability of cotton fabrics: synthesis, characterization and flammability. Chem Eng J. 2016;294:167–75.
Shen D, Xiao R, Gu S, Luo K. The pyrolytic behavior of cellulose in lignocellulosic biomass: a review. RSC Adv. 2011;1:1641–60.
Zhao B, Chen L, Long JW, Chen HB, Wang YZ. Aluminum hypophosphite versus alkyl-substituted phosphinate in polyamide 6: flame retardance, thermal degradation, and pyrolysis behavior. Ind Eng Chem Res. 2013;52:2875–86.
Illy N, Fache M, Menard R, Negrell C, Caillol S, David G. Phosphorylation of bio-based compounds: state of the art. Polym Chem. 2015;6:6257–91.
Yuan B, Bao C, Guo Y, Song L, Liew KM, Hu Y. Preparation and characterization of flame retardant aluminum hypophosphite/poly (vinyl alcohol) composite. Ind Eng Chem Res. 2012;51:14065–75.
Pan H, Wang W, Pan Y, Song L, Hu Y, Liew KM. Formation of self-extinguishing flame retardant biobased coating on cotton fabrics via layer by layer assembly of chitin derivatives. Carbohyd Polym. 2015;115:516–24.
Xiao S, Chen M, Dong L, Deng C, Chen L, Wang Y. Thermal degradation, flame retardancy and mechanical properties of thermoplastic polyurethane composites based on aluminum hypophosphite. Chin J Polym Sci. 2014;32:98–107.
Dong C, Lu Z, Zhu P, Zhang F, Zhang X. Combustion behaviors of cotton fabrics treated by a novel guanidyl and phosphorus containing polysiloxane flame retardant. J Therm Anal Calorim. 2015;119:349–57.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Furtana, S., Mutlu, A. & Dogan, M. Thermal stability and flame retardant properties of calcium- and magnesium-hypophosphite-finished cotton fabrics and the evaluation of interaction with clay and POSS nanoparticles. J Therm Anal Calorim 139, 3415–3425 (2020). https://doi.org/10.1007/s10973-019-08751-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08751-2