Abstract
Four novel polyhedral oligomeric silsesquioxanes/polystyrene (POSS/PS) nanocomposites, having formula R7R′1(SiO1.5)8/PS (where R = C6H5– and R′ = p-C6H4-X, with X = –CH3, –OCH3, –F, –Cl) were synthesized by in situ polymerization of styrene, in the presence of 5% w/w of POSS. The obtained nanocomposites were characterized by 1H-NMR spectroscopy and by the glass transition temperature (Tg) determination. The thermal degradations of nanocomposites were thus carried out in thermobalance, in the scanning mode, in the temperature range r.t.—700 °C, in both flowing nitrogen and static air atmospheres. Temperature at 5% mass loss (T5%) was determined as parameter measuring the resistance to thermal degradation. The obtained T5% values of nanocomposites were largely higher than those found for PS, which was also degraded for comparison in the same experimental conditions, thus indicating higher resistance to thermal degradation in respect to neat polymer. Also, the sameness of nanocomposites T5% values was interpreted and explained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymers are a great and very important category of organic compounds that have changed our lifestyle. They have interesting properties, but often exhibit modest physical and mechanical characteristics. Obviously, polymers having good thermal and mechanical characteristics have been synthesized, but their cost is often high. The chemical modification of low-cost polymers to improve them from mechanical or thermal point of view has thus been investigated through property-structure studies and many works in this field are reported in the literature [1,2,3,4].
Alternatively, another interesting way to improve the properties of polymers is mixing them with opportunely selected materials, so the research in this field has been also largely developed and it has been reported in the literature that the physical, mechanical and thermal properties of polymers are usually improved by adding to them inorganic materials [5,6,7]. Moreover, the dispersion into polymer of inorganic substances of nanometric size results usually in the consistent enhancement of physical, mechanical, barrier and flammability properties of obtained nanocomposites if compared with those of neat polymer or those of conventionally filled polymer composites [8, 9]. Also, nanocomposites show usually a large increase in thermal stability. This last property is important because these products can be subjected to high temperatures during processing or in service [10, 11].
Several categories of inorganics, such as layered silicates, or organic compounds, such as carbon nanotubes or others, have been tested in the past as fillers to obtain polymer-based nanocomposites [12, 13]. More recently, a relatively novel class of inorganic–organic compounds, namely polyhedral oligomeric silsesquioxanes (POSSs), has been employed to this use. The general formula of POSSs is (RSiO1.5)n, with n = 6, 8, 10 or 12. The POSSs most used as fillers for polymer-based nanocomposites have n = 8. So a typical POSS particle is formed by an inorganic Si8O12 skeleton, surrounded by eight identical or different organic groups, as alkyl, aryl or any of their derivatives, linked with covalent bonds to silicon atoms [14, 15].
A single POSS molecule has highly symmetric structure and a diameter of 1–3 nm, the organic groups linked to cage silicon atoms included. This compact structure gives to POSSs high thermal and chemical stabilities, that allow their use in various fields, as, for example, high performance materials [16, 17], flame retardants [18, 19], homogeneous POSSs-supported catalysts [20, 21] and applications in proton exchange membranes [22, 23]. Due to the Si–O–Si bonds, POSSs exhibit biodegradability and are also used in biological and biomedical fields [24,25,26,27].
POSSs can be incorporated in host polymers by several methods, copolymerization [28], grafting [29], blending [30], in situ polymerization [31] or others, thereby offering to scientists special opportunities to prepare new thermoset [32] or thermoplastic [33] materials, the choice of method being depending on the particular characteristics of polymer and POSS. Nevertheless, particular attention must be devoted to selecting POSSs as fillers for polymer-based nanocomposites. Moreover, if we want obtain to products showing characteristics better than those of neat polymers, fillers must be nanometrically dispersed in polymer matrices. From this viewpoint, it is worth to consider that the nature of side groups in POSSs molecules largely affects their miscibility with polymers [34, 35], thus determining a higher or lower nanometric dispersion into host matrix [36, 37]. In particular, it has been reported in the literature that the presence of aliphatic groups determines the enhancement of their solubility in common solvents and compatibility with host polymer but worsens the thermal properties, while the presence of aromatic groups acts in the opposite way [38]. The inorganic core formed by the silicon atoms confers rigidity, thermal stability and resistance to oxidation [39] to POSS molecules. Since the over-reported characteristics are all requested for the POSSs to be employed as fillers in nanocomposites, research has been developed toward the synthesis of novel thermally highly stable POSSs having good miscibility with host polymers, and then guaranteeing excellent nanometric dispersion. This result can be obtained by balancing opportunely aliphatic and aromatic groups in silicon cage. Another factor largely affecting POSSs solubility as well as the miscibility with polymers is their more or less symmetric structure. In particular, it has been demonstrated that more elevated symmetry levels of POSSs correspond to lower solubility and miscibility and vice versa [34, 39].
Our group started several years ago, and has still in progress, a wide research having the goal of the synthesis and characterization of new thermally stable POSSs-based nanocomposites, with Polystyrene (PS) as polymer matrix. In a recent work [35] we published the results of a study concerning the thermal and oxidative degradations of four novel hybrid inorganic/organic POSSs having seven C6H5 groups linked to seven silicon atoms of cage, while a substituted –p(C6H4X) group (X = –CH3, –OCH3, –F, –Cl) was linked to the eighth silicon atom. The studied POSSs were the following: (C6H5)7 (p-C6H4–CH3) (SiO1.5)8, (C6H5)7 (p-C6H4–OCH3) (SiO1.5)8, (C6H5)7 (p-C6H4–F) (SiO1.5)8 and (C6H5)7 (p-C6H4–Cl) (SiO1.5)8. The aim of that work was to investigate the effect on the thermal stability and solubility of POSS in common solvents, due to the reduction of symmetry level determined to the substitution of only one hydrogen atom of silicon cage. The results obtained with the over-reported monosubstituted POSSs were compared with those of highly symmetric, thermally very stable and insoluble unsubstituted octaphenyl POSS. Mono-substituted POSSs exhibited higher solubility and slightly lower resistance to the thermal degradation than octaphenyl one. This behavior was attributed to the reduction of symmetry level due to the substitution of one hydrogen atom of a POSS phenyl group with substituents of different size [35].
Due to these encouraging results, in the present work, we would verify the possibility to prepare, by in situ polymerization of styrene, PS nanocomposites with the above-described octaphenyl POSSs. Nanoparticles concentration of 5% w/w was chosen on the basis of our previous investigations that suggested higher thermal performance for PS nanocomposites at this concentration [5, 37, 40]. Additional aim of this work was also to investigate if and how much the obtained nanocomposites have better thermal properties than neat PS and nanocomposites, previously synthesized by our group, with less than eight phenyl groups at the periphery of the silicon cage. The products synthesized are listed below:
4-Methylphenyl heptaphenyl POSS/PS | 1 |
4-Methoxyphenyl heptaphenyl POSS/PS | 2 |
4-Fluorophenyl heptaphenyl POSS/PS | 3 |
4-Chlorophenyl heptaphenyl POSS/PS | 4 |
Obtained nanocomposites were first characterized by 1H NMR spectroscopy and by the determination of glass transition temperature (Tg) performed through Differential Scanning Calorimetry (DSC). The resistance to the thermal degradation was checked through the determination of two parameters associated with the thermal stability, namely temperature at 5% mass loss (T5%) and solid residue at 700 °C in both inert (flowing nitrogen) and oxidative (static air) atmosphere. These values were determined by thermogravimetry (TG), in dynamic heating conditions. Finally, the solid residues at 700 °C obtained at the end of TG experiments were analyzed by FTIR spectroscopy.
Experimental
Materials
Styrene and 2,2-azobis (isobutyronitrile) (AIBN) was purchased from Aldrich Co. Both the substances were purified: Styrene in an inhibitor removal column, while AIBN was re-crystallized twice from dry ethanol, at a temperature < 40 °C and out of the direct light. Toluene was purchased from Aldrich Co., stirred for 24 h over CaH2 and distilled in nitrogen atmosphere. 4-methylphenyl (1), 4-methoxyphenyl (2), 4-fluorophenyl (3) and 4-chlorophenyl (4) heptaphenyl POSSs were synthesized according with the procedure set up by our research group and reported elsewhere [35]. The structures of the prepared POSS molecules are reported in Table 1.
In situ free-radical polymerization, by using a mixture of 5% w/w POSS/styrene, was carried out in toluene following the procedure reported below for sample 2, as example. 0.20 g of 4-methoxyphenyl heptaphenyl POSSs and 3.80 g of styrene were dissolved in 40 mL of toluene; to the obtained mixture were added 12 mg of AIBN and then it was frozen in a liquid nitrogen bath, degassed with a vacuum pump, and finally thawed. After repeating this procedure three times, the tube, sealed under vacuum, was heated at 70 °C for 24 h under stirring. The obtained clear solution was purified in methanol (500 mL) and the nanocomposite precipitated and collected by filtration. Finally, the sample was dried under vacuum at 40 °C obtaining a yield of 3.42 g (85%). PS and compounds 1 (yield 70%), 3 (yield 77%) and 4 (yield 81%) were prepared with the same polymerization procedure.
1H NMR spectroscopy
1H NMR spectroscopy was carried out in a Varian Unity Inova spectrometer (1H 500 MHz) by using CDCl3 as solvent and tetramethylsilane (TMS) as internal standard. The exact filler content was calculated thanks to the ratio between the POSS aromatic hydrogen atoms and the PS hydrogen ones in the 1H NMR spectra. This procedure allowed us to determine 7.12, 5.25, 6.67 and 5.82% for sample 1, 2, 3 and 4, respectively.
DSC measurements
A Mettler DSC 1 Star System was used to carry out the calorimetric investigation to determine the glass transition temperatures (Tg) of the investigated materials. The instrument was calibrated in enthalpy and temperature following the procedure developed by us in the past [41]. The agreement with the literature standard of the enthalpy was within 0.25% [42] after calibration with indium, while the agreement of temperature was within 0.08% in respect to their literature values [42] after several calibration scans with indium and tin. Measurements were performed from room temperature up to 250 °C, at a heating rate of 10 °C min−1, on samples of about 5.0 × 10−3 g, held in sealed aluminum crucibles.
Thermogravimetric analysis
Thermal degradations of the synthesized nanocomposites were performed in a Mettler Thermogravimetric Analyzer TGA 1 Star System. The temperature calibration was made according to the suggestions of the equipment manufacturer, take advantage of magnetic properties change of three metal standards (Isatherm, Nickel-alloy and Trafoperm 86) at their Curie points (148, 355 and 750 °C, respectively). TGA scans were carried out at 10 °C min−1, in the temperature range 25–700 °C, in both flowing nitrogen (0.02 L min−1) and static air atmosphere. About 5 × 10−3 g of sample were degraded into open alumina crucibles. In order to correct the error in the mass determination due to the reduction of the buoyancy force on increasing temperature [43], a TG scan with an empty alumina crucible (blank) was preliminarily carried out in the same experimental conditions described above. The so obtained blank curve was subtracted from those of samples, obtaining corrected degradation TG curves. At the end of each experiment, these data were used to plot the percentage of undegraded sample, (1 − D)%, as a function of temperature, where D = (Wo − W)/Wo, and Wo and W were the masses at the starting point and during experiment, respectively.
IR spectroscopy
A Perkin Elmer Spectrum 100 spectrometer was employed in the determination of the Fourier Transform Infrared (FTIR) spectra of the solid residues obtained from the TGA experiments. Spectra were carried out directly on samples at r.t., without any preliminary treatment, using an universal ATR sampling accessory, from 4000 to 600 cm−1, with a resolution of 4.0 cm−1.
Results and discussion
The 1–4 nanocomposites were prepared by in situ polymerization of polystyrene in the presence of about 5% of POSS, as described in details in the Experimental section.
The nanocomposites obtained were analyzed by 1H NMR spectroscopy, in order to determine the POSS percentage in the prepared products. The determined percentages of filler in various nanocomposites are reported in Table 2. These filler contents, slightly higher than those of starting mixtures, were probably due to the formation, during the in situ polymerization, of methanol soluble oligomers of polystyrene [44].
The glass transition temperatures (Tg) of 1–4 nanocomposites as well as that of PS were thus calorimetrically determined, and are reported in Table 2 too.
The thermal degradations of 1–4 nanocomposites were then carried out in the crucible of our thermobalance, in dynamic heating conditions, in the temperature range 25–700 °C, in both inert (flowing nitrogen) and oxidative (static air) atmospheres, at the heating rate of 10 °C min−1. This scanning rate was selected because it is a medium value among those normally used in the experiments of polymer thermal degradation in the scanning mode.
The main parameter determined to evaluate higher or lower resistance to thermal degradation of our samples was the temperature at 5% mass loss, whose value was drawn directly from the list of data of thermobalance processor.
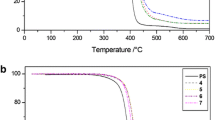
T5% values were determined in the place of initial decomposition temperature (Ti) because in our opinion they are more reliable. Moreover, Ti values, which are graphically determined by the TG degradation curves, are largely depending on the slope of the descending piece of curves. The degradations in inert environment of 1–4 PS nanocomposites were first carried out and the corresponding TG and DTG curves are reported in Figs. 1 and 2, together with that of neat PS, which was performed for comparison.
In Table 2 the obtained T5% values together with the temperatures at maximum rate of mass loss (Tm) ones for PS and various nanocomposites are listed. The degradations in oxidative atmosphere were thus carried out, and the obtained TG and DTG curves are reported in Figs. 3 and 4, the corresponding T5% values are reported in Table 2.
In both investigated environments net PS degraded completely, while all nanocomposites evidenced the formation of a little amount of residue stable up to 700 °C (Table 2). The solid residues obtained by the thermal and thermo-oxidative degradations of our compounds were investigated by FTIR Spectroscopy, in order to find, if possible, useful information about the nature of degradation products.
The obtained experimental data suggest some considerations:
-
the T5% values of all prepared nanocomposites in both investigated environments are much higher than those of neat PS. The ΔT5% values (ΔT5% = T5% of nanocomposite − T5% of PS), that represent substantially a measure of the increment of the resistance to thermal degradation due to the introduction of POSSs in polymer matrix, were particularly elevated, thus indicating a high increment of thermal stability of various nanocomposites in respect to neat PS;
-
the same trend observed for the temperature at 5% of mass loss was recorded for the temperatures at maximum rate of mass loss (Tm), thus confirming the enhancement of the resistance to the thermal degradation of the polymer reinforced with POSSs;
-
as shown in Fig. 5 and differently than T5% the addition of POSSs to PS appears do not affect substantially the glass transition temperature (average ΔTg ~ 1 °C);
-
the T5% values of all prepared nanocomposites were substantially the same, independently on the nature of the substituent introduced in the p-position of POSS phenyl group. This behavior is different than that observed by us in the past on similar compounds [45, 46]. Then the question: why? In order to answer this question, we analyzed the differences among the structures of various compounds. The POSSs present in the nanocomposites here studied show height phenyl groups linked to a silicon cage (Table 1). In the POSSs of the nanocomposites previously investigated only one phenyl group was linked to the silicon cage, while seven isobutyl or cyclopentyl groups are placed in the other seven positions of the cage. This one is, in our opinion, the key to interpret the different behavior observed, on considering that the thermal stability is affected by the nanometric dispersion of POSS in polymer matrix [36]. The POSSs used for the nanocomposites here investigated can be considered obtained from the very symmetric octaphenyl POSS by the substitution of the H in the para position of phenyl group with a single atom (F, Cl) or with a little single group (CH3, O–CH3). The consequent modification of POSS symmetry appears similar for the four compounds. The increment of nanocomposite thermal stability is connected with the level of filler dispersion in polymer matrix, which depends on the POSS symmetry [36, 37]. As a consequence, the T5% values of the investigated nanocomposites are very similar. Also, the Tg values trend remains practically constant (0.2 °C ≤ ΔTg ≤ 1.9 °C), according to the literature evidence reporting that symmetric octa-substituted POSS, in which stronger POSS–POSS interactions occur, giving rise to lower dispersion of filler into polymeric matrix and thus a reduce, or in staying constant, of viscosity [36, 47].
In the heptacyclopentyl, mono phenyl- and heptaisobutyl, mono phenyl- POSSs previously studied [45, 46], the substitution of the H of phenyl group occurred on not perfectly symmetric basic compounds, so giving rise to different modifications of symmetry among various POSSs. It could explain, in our opinion, the differences observed among various groups of nanocomposites studied in the past;
-
differently from PS that degraded completely, the degradation TG curves evidenced, for all studied 1–4 nanocomposites, in both studied environments, the formation of little amounts of residue stable up to 700 °C (Table 2). The residues found were analyzed by FTIR spectroscopy, and the obtained spectra did not exhibit any substantial difference with each other, showing only silica IR bands. In Fig. 6 the FTIR spectra of the residues from the thermal and thermo-oxidative degradations of nanocomposite 1 are reported as an example.
Conclusions
The results reported in this paper show clearly that PS-based nanocomposites having as reinforcement monosubstituted octaphenyl POSSs can be easily prepared by in situ polymerization of styrene in the presence of 5% w/w of POSS. In fact the slight modification of the POSS molecules symmetry, by the introduction of fluorine and chlorine atoms and methyl and methoxy groups in the place of a hydrogen atom, lead to an improvement of monosubstituted octaphenyl POSS miscibility in the matrix in respect to the unsubstituted octaphenyl POSS, for which we could not prepare the corresponding nanocomposite. This increased solubility of the silicon nanoparticles in the polymer matrix, has allowed for the first time, to the best of our knowledge, the synthesis of polystyrene reinforced with octaphenyl POSS. In the obtained hybrid nanocomposites, the filler concentration was slightly higher than that present in the reactant mixtures and all prepared materials exhibit a large improvement of thermal stability in respect to neat PS, as supported by the values of T5%. Thermogravimetric and IR spectroscopic data revealed the presence of a stable residue, of siliceous nature, thus confirming the reinforcement effect of POSS, on the polymer matrix.
References
Kaszas G. Basic physical properties/structure of polystyrene–polyisobutylene–polystyrene triblock copolymers. Polym Mater Sci Eng Proc ACS Div Polym Mater Sci Eng. 1993;68:325–6.
Bunz UHF. Poly(aryleneethynylene)s: syntheses, properties, structures, and applications. Chem Rev. 2000;100(4):1605–44.
Cavallaro G, De Lisi R, Lazzara G, Milioto S. Polyethylene glycol/clay nanotubes composites: thermal properties and structure. J Therm Anal Calorim. 2013;112(1):383–9.
Catauro M, Dell’Era A, Vecchio Ciprioti S. Synthesis, structural, spectroscopic and thermoanalytical study of sol-gel derived SiO2–CaO–P2O5 gel and ceramic materials. Thermochim Acta. 2016;625:20–7.
Blanco I, Bottino FA, Cicala G, Latteri A, Recca A. A kinetic study of the thermal and thermal oxidative degradations of new bridged POSS/PS nanocomposites. Polym Degrad Stabil. 2013;98(12):2564–70.
Duce C, Vecchio Ciprioti S, Ghezzi L, Ierardi V, Tinè MR. Thermal behavior study of pristine and modified halloysite nanotubes: a modern kinetic study. J Therm Anal Calorim. 2015;121(3):1011–9.
Catauro M, Bollino F, Papale F, Gallicchio M, Pacifico S. Influence of the polymer amount on bioactivity and biocompatibility of SiO2/PEG hybrid materials synthesized by sol-gel technique. Mater Sci Eng C. 2015;48:548–55.
Zhang W, Camino G, Yang R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: an overview of fire retardance. Progr Polym Sci. 2017;67:77–125.
Morici E, Di Bartolo A, Arrigo R, Dintcheva NT. POSS grafting on polyethylene and maleic anhydride-grafted polyethylene by one-step reactive melt mixing. Adv Polym Tech. 2018;37(2):349–57.
Abate L, Blanco I, Bottino FA, Di Pasquale G, Fabbri E, Orestano A, Pollicino A. Kinetic study of the thermal degradation of PS/MMT nanocomposites prepared with imidazolium surfactants. J Therm Anal Calorim. 2008;91(3):681–6.
Leszczyńska A, Stafin K, Pagacz J, Mičušík M, Omastova M, Hebda E, Pielichowski J, Borschneck D, Rose J, Pielichowski K. The effect of surface modification of microfibrillated cellulose (MFC) by acid chlorides on the structural and thermomechanical properties of biopolyamide 4.10 nanocomposites. Ind Crops Prod. 2018;116:97–108.
Cavallaro G, Lazzara G, Konnova S, Fakhrullin R, Lvov Y. Composite films of natural clay nanotubes with cellulose and chitosan. Green Mater. 2014;2(4):232–42.
Massaro M, Lazzara G, Milioto S, Noto R, Riela S. Covalently modified halloysite clay nanotubes: synthesis, properties, biological and medical applications. J Mater Chem B. 2017;5(16):2867–82.
Harrison PG. Silicate cages: precursors to new materials. J Organomet Chem. 1997;542(2):141–83.
Baney RH, Itoh M, Sakakibara A, Suzuki T. Silsesquioxanes. Chem Rev. 1995;95(5):1409–30.
Illescas S, Sánchez-Soto M, Milliman H, Schiraldi DA, Arostegui A. The morphology and properties of melt-mixed polyoxymethylene/monosilanolisobutyl-POSS composites. High Perform Polym. 2011;23(6):457–67.
Tanaka K, Chujo Y. Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J Mater Chem. 2012;22:1733–40.
Fina A, Abbenhuis HCL, Tabuani D, Camino G. Metal functionalized POSS as fire retardants in polypropylene. Polym Degrad Stabil. 2006;91(10):2275–81.
Wang X, Hu Y, Song L, Xing W, Lu H. Thermal degradation behaviors of epoxy resin/POSS hybrids and phosphorus–silicon synergism of flame retardancy. J Polym Sci B Polym Phys. 2010;48:693–705.
Zhang Y, Ye Z. Homogeneous polyhedral oligomeric silsesquioxane (POSS)-supported Pd-diimine complex and synthesis of polyethylenes end-tethered with a POSS nanoparticle via ethylene living polymerization. Chem Commun. 2010;2008(10):1178–80.
Zhang Y, Ye Z. Covalent surface grafting of branched polyethylenes on silica nanoparticles by surface-initiated ethylene living polymerization with immobilized 98 Pd-diimine catalysts. Macromolecules. 2008;41:6331–8.
Cheng CC, Yen YC, Ko FH, Chu CW, Fan SK, Chang FC. A new supramolecular film formed from a silsesquioxane derivative for application in proton exchange membranes. J Mater Chem. 2012;22:731–4.
Choi J, Lee KM, Wycisk R, Pintauro PN, Mather PT. Sulfonated polysulfone/POSS nanofiber composite membranes for PEM fuel cells. J Electrochem Soc. 2010;157:B914–9.
Rathbone S, Furrer P, Lubben J, Zinn M, Cartmell S. Biocompatibility of polyhydroxyalkanoate as a potential material for ligament and tendon scaffold material. J Biomed Mater Res Part A. 2010;93:1391–403.
Guo YL, Wang WS, Otaigbe JU. Biocompatibility of synthetic poly(ester urethane)/polyhedral oligomeric silsesquioxane matrices with embryonic stem cell proliferation and differentiation. J Tissue Eng Regener Med. 2010;4:553–64.
Ghanbari H, Kidane AG, Burriesci G, Ramesh B, Darbyshire A, Seifalian AM. The anti-calcification potential of a silsesquioxane nanocomposite polymer under in vitro conditions: potential material for synthetic leaflet heart valve. Acta Biomater. 2010;6:4249–60.
Blanco I. Polyhedral oligomeric silsesquioxanes (POSS)s in medicine. J Nanomed. 2018;1(1):1–3.
Wang X, Yang YK, Yang ZF, Zhou XP, Liao YG, Lv CC, Chang FC, Xie XL. Thermal properties and liquid crystallinity of side-chain azobenzene copolymer containing pendant polyhedral oligomeric silsequioxanes. J Therm Anal Calorim. 2010;102:739–44.
Devaraju S, Vengatesan MR, Selvi M, Alagar M. Thermal and dielectric properties of newly developed linear aliphatic-ether linked bismaleimide-polyhedral oligomeric silsesquioxane (POSS-AEBMI) nanocomposites. J Therm Anal Calorim. 2014;117:1047–63.
Tanaka K, Adachi S, Chujo Y. Structure–property relationship of octa-substituted POSS in thermal and mechanical reinforcements of conventional polymers. J Polym Sci Part A Polym Chem. 2009;47:5690–7.
Wu Q, Zhang C, Liang R, Wang B. Combustion and thermal properties of epoxy/phenyltrisilanol polyhedral oligomeric silsesquioxane nanocomposites. J Therm Anal Calorim. 2010;100:1009–15.
Gao J, Zhu FL, Yang J, Liu X. Synthesis and curing kinetics of UV-curable waterborne bisphenol-s epoxy-acrylate/polyurethane-acrylate/methylacryloylpropyl-POSS nanocomposites. J Macromol Sci Part B Phys. 2014;53:1800–13.
Xia L, Li F, Shentu B, Weng Z. Thermal degradation behavior and flame retardancy of polycarbonate containing poly[(phenylsilsesquioxane)-co-(dimethylsiloxane)] and potassium diphenyl sulfonate. J Macromol Sci Part B Phys. 2013;52:310–8.
Blanco I, Bottino FA, Abate L. Influence of n-alkyl substituents on the thermal behaviour of polyhedral oligomeric silsesquioxanes (POSSs) with different cage’s periphery. Thermochim Acta. 2016;623:50–7.
Blanco I, Bottino FA, Abate L. Mono substituted octaphenyl POSSs: the effects of substituents on thermal properties and solubility. Thermochim Acta. 2017;655:117–23.
Blanco I, Bottino FA, Bottino P. Influence of symmetry/asymmetry of the nanoparticles structure on the thermal stability of polyhedral oligomeric silsesquioxane/polystyrene nanocomposites. Polym Compos. 2012;33(11):1903–10.
Blanco I, Bottino FA, Cicala G, Cozzo G, Latteri A, Recca A. Synthesis and thermal characterization of new dumbbell shaped POSS/PS nanocomposites: influence of the symmetrical structure of the nanoparticles on the dispersion/aggregation in the polymer matrix. Polym Compos. 2015;36(8):1394–400.
Fina A, Tabuani D, Carniato F, Frache A, Boccaleri E, Camino G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim Acta. 2006;440(1):36–42.
Moore BM, Ramirez SM, Yandek GR, Haddad TS, Mabry JM. Asymmetric aryl polyhedral oligomeric silsesquioxanes (ArPOSS) with enhanced solubility. J Organomet Chem. 2011;696:2676–80.
Blanco I, Bottino FA. Effect of the substituents on the thermal stability of hepta cyclopentyl, phenyl substitued-polyhedral oligomeric silsesquioxane (hcp-POSS)/polystyrene (PS) nanocomposites. AIP Conf Proc. 2012;1459(1):247–9.
Badea E, Blanco I, Della Gatta G. Fusion and solid-to-solid transitions of a homologous series of alkane-α, ω-dinitriles. J Chem Thermodyn. 2007;39(10):1392–8.
Della Gatta G, Richardson MJ, Sarge SM, Stølen S. Standards, calibration, and guidelines in microcalorimetry. Part 2. Calibration standards for differential scanning calorimetry (IUPAC technical report). Pure Appl Chem. 2006;78(7):1455–76.
Blanco I, Cicala G, Latteri A, Saccullo G, El-Sabbagh AMM, Ziegmann G. Thermal characterization of a series of lignin-based polypropylene blends. J Therm Anal Calorim. 2017;127(1):147–53.
Blanco I, Abate L, Bottino FA, Chiacchio MA. Synthesis and thermal behaviour of novel aliphatic/aromatic hepta-cyclopentyl bridged polyhedral oligomeric silsesquioxanes (POSSs)/polystyrene (PS) nanocomposites. J Inorg Organomet Polym Mater. 2015;25(6):1456–64.
Blanco I, Abate L, Bottino FA. Variously substituted phenyl hepta cyclopentyl-polyhedral oligomeric silsesquioxane (ph, hcp-POSS)/polystyrene (PS) nanocomposites: the influence of substituents on the thermal stability. J Therm Anal Calorim. 2013;112(1):421–8.
Blanco I, Abate L, Antonelli ML, Bottino FA, Bottino P. Phenyl hepta cyclopentyl—polyhedral oligomeric silsesquioxane (ph, hcp-POSS)/polystyrene (PS) nanocomposites: the influence of substituents in the phenyl group on the thermal stability. eXPRESS Polym Lett. 2012;6(12):997–1006.
Blanco I. The rediscovery of POSS: a molecule rather than a filler. Polymers. 2018;10(8):904.
Acknowledgements
Ignazio Blanco is grateful to the MIUR for the grant “Fund for basic research activities,” and to the University of Catania within the “Piano della Ricerca Dipartimentale 2016-2018” of the Department of Civil Engineering and Architecture, for supporting the project MATErials LIfe foreCAst (MATELICA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blanco, I., Abate, L., Bottino, P. et al. Synthesis and thermal characterization of monosubstituted octaphenyl POSS/polystyrene nanocomposites. J Therm Anal Calorim 138, 2357–2365 (2019). https://doi.org/10.1007/s10973-019-08212-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08212-w