Abstract
A series of novel hepta cyclopentyl bridged polyhedral oligomeric silsesquioxanes (POSSs)/polystyrene (PS) nanocomposites, at different POSS contents (1, 3 and 5 % w/w), was synthesized and characterized. Nanocomposites were prepared by in situ polymerization of styrene in the presence of POSS which has not polymerizable groups, aiming to obtain well-dispersed POSS/PS systems. The actual filler concentration in the obtained nanocomposites was checked by 1 H NMR and FTIR spectroscopy. Degradations were carried out into a thermobalance at various heating rates in flowing nitrogen and in a static air atmosphere, and the characteristic parameters of thermal stability, namely temperature at 5 % mass loss (T 5 %) and activation energy (E a) of degradation, of the various nanocomposites were determined. We investigated the influence of the nature of POSSs cage’s periphery on the thermal stability of the obtained materials, and with this aim, we compared the properties of hepta cyclopentyl bridged POSS/PS nanocomposites with those of similar hepta isobutyl bridged POSS/PS ones previously studied. The obtained results were discussed and interpreted.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last 30 years, the increasing use of polymers in structural applications has required an increase in their thermal and mechanical response and the use of fillers is a simple and effective strategy to satisfy this demand. Taking a cue from one of nature’s remarkable features, that is the ability to combine at the nanoscale organic and inorganic components thus allowing the making of smart natural materials, scientists have investigated the possibility of using hybrid materials that found a perfect compromise between different properties or functions, thus leading to the development of innovative industrial applications. These new generations of hybrid materials, born from the very fruitful activities in this research field, will open a land of promising applications in many areas: optics, electronics, ionics, mechanics, energy, environment, biology, medicine, fuel and solar cells, catalysts, sensors, etc. [1]. The mixing of polymers and nanoparticles, by controlling their spatial distribution into the matrix, is opening pathways for materials with enhanced macroscopic performance. Due to its dual nature, the well-defined rigid structure of the siliceous core and the nanometer size scale, polyhedral oligomeric silsesquioxanes (POSSs) have climbed, in recent years, the rankings of the most used nanoreinforcements. POSS molecules consist of a polyhedral inorganic core, typically Si8O12 with a 0.53 nm diameter, surrounded by organic chains attached to the vertices. A short or long inert organic chain may be chosen to improve solubility in the matrix, while one or more suitable reactive groups may enable the POSS units to bond on the macromolecular structure itself [2]. Various methods of preparing these nanostructured materials are known, ranging from chemical tethering (e.g., grafting and copolymerization reactions) [3–5] to physical blending (e.g., solution blending and melt mixing) [6, 7], which would result in the enhancement of the polymer properties including the reduction in flammability and dielectric constant and increase in thermal and mechanical properties [3, 8].
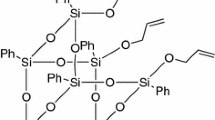
In this context it is included the work that our group has carried out, in recent years, on the synthesis of POSSs [9–11] to be used for the improvement of thermal properties of polystyrene (PS), one of the most mass-productive and commercialized polymers but with low glass transition temperatures (T g) and relatively poor thermal stabilities, for further applications in severer situations [12]. Since the effective modification of polymer with POSSs depends, to a large extent, on the successful dispersion of the nanoparticles within the matrix, we studied, in these years, the influence of filler percentage [13], of the electron-donor or electron-withdrawing character of the substituents on silicon cage [14, 15], of symmetry/asymmetry [16] and rigidity/flexibility [17–19] of the nanoparticles structure on the dispersion/aggregation in the polymer matrix. On continuing the study in this field, we report here the synthesis and thermal behavior of a bridged POSS/PS system whose molecular structure is reported in Fig. 1, wherein the seven isobutyl groups attached to silicon cage of compounds of Ref. [19] have been replaced with seven cyclopentyl groups. The aim was to investigate whether and how much the nature of the organic periphery influence the compatibility with the matrix, thus enhancing the thermal stability of the obtained nanocomposites, and to confirm the 5 % as the best filler percentage.
The studied nanocomposites, synthesized by in situ polymerization of styrene in the presence of POSS which has not polymerizable groups, aiming to obtain well-dispersed POSS/PS systems, are the following, and will be indicated in the text by the corresponding numbers, as follows:
- 1,2-bis(heptacyclopentyl-T8-silsesquioxyl)ethane (POSS)/PS at 1 % w/w:
-
1
- 1,2-bis(heptacyclopentyl-T8-silsesquioxyl)ethane (POSS)/PS at 3 % w/w:
-
2
- 1,2-bis(heptacyclopentyl-T8-silsesquioxyl)ethane (POSS)/PS at 5 % w/w:
-
3
where the 1, 3 and 5 % values represent the filler percentages in the reactant mixtures.
The characterization of PS and obtained nanocomposites was made by 1 H NMR spectroscopy to check the actual filler content and FT-IR spectroscopy. Thermal and thermoxidative degradations were studied by thermogravimetric (TG) analysis, and the parameters associated, namely temperature at 5 % mass loss (T 5 %) and apparent activation energy (E a) of degradation, were determined and compared with each other and with those of neat polymer and with those of the corresponding hepta isobutyl POSSs-based nanocomposites previously studied. Finally, glass transition temperatures were determined by differential scanning calorimetry (DSC).
Experimental
Materials
Styrene and 2,2-azobis(isobutyronitrile) (AIBN) were purchased by Aldrich Co., the first one was purified in an inhibitor removal column, while AIBN was recrystallized twice from dry ethanol at temperatures less than 40 °C and out of direct light. Toluene was stirred over calcium hydride for 24 h and distilled in a nitrogen atmosphere. 1,2-bis(heptacyclopentyl-T8-silsesquioxyl)ethane (Fig. 1) was synthesized by corner capping reaction and characterized as described elsewhere [11].
Nanocomposites were obtained by in situ polymerization of styrene and 1,2-bis(heptacyclopentyl-T8-silsesquioxyl)ethane mixtures, at 1, 3 and 5 % w/w, respectively, in toluene. The details of free-radical polymerization procedure are described, as an example, for compound 3. After the dissolution of styrene (3,80 g) and 1,2-bis(heptacyclopentyl-T8-silsesquioxyl)ethane (0,20 g) in 40 mL of toluene, AIBN radical initiator (12 mg) was added; the mixture was frozen in a liquid nitrogen bath, degassed with a vacuum pump and then thawed. This operation was repeated three times and then the tube, sealed under vacuum, was heated at 70 °C for 24 h under stirring. The clear solution was poured into a large excess of methanol (600 mL), and the precipitated nanocomposite was collected by filtration and dried under vacuum at 40 °C. The yield was 3.64 g (91 %). The same polymerization procedure was used to prepare neat PS and compounds 1 (yield 88 %) and 2 (yield 86 %).
1H NMR spectroscopy
1 H NMR spectra were recorded by a Varian Unity Inova instrument (1 H 500 MHz), using CDCl3 as solvent and TMS as internal standard. The 1 H NMR spectra of all compounds are very similar with each other and show peaks attributable to the aromatic protons of styrene units as broad signal centered at 7.08 δ (3H) and at 6.51 δ (2H) and to the aliphatic protons at 1.86 δ (1H) and 1.48 δ (2H). The signals of the protons of cyclopentyl-POSS filler [11], partially covered by the strong signals of PS except for the protons of the cyclopentyl ring carbon attached to Si atom, appear at 0.96 δ. This experimental evidence confirms the presence of filler in composites and allows us to calculate with a good approximation its percentage, through the ratio aromatic protons of PS and protons at 0.96 δ of the filler.
IR Spectroscopy
Fourier transform infrared (FTIR) spectroscopy was performed with a PerkinElmer Spectrum 100 spectrometer, using a universal ATR sampling accessory. Spectra were recorded at r.t. from 4000 to 650 cm−1, with a resolution of 4.0 cm−1, directly on compounds, without any preliminary treatment.
Thermogravimetric analysis
A Mettler Thermogravimetric Analyzer TGA 1 Star System was used for the evaluation of thermal and thermal oxidative degradations of the studied compounds. The temperature calibration of equipment was made according to the method suggested by Mettler, which is based on the change of magnetic properties of three metal samples (Isatherm, Nickel and Trafoperm) at their Curie points (148, 355 and 750 °C, respectively). Degradations were carried out into open alumina crucibles, in dynamic heating conditions, at various heating rates (Φ = 2, 5, 7.5, 10, 12.5, 15, 17.5 and 20 °C min−1), in the temperature range 25–700 °C, in both flowing nitrogen (0.02 L min−1) and static air atmosphere. Samples of about 5 × 10−3 g were used for the degradation experiments, and their masses were measured as a function of temperature. A thermogravimetric (TG) run with an empty pan (blank) was preliminarily performed, in the same experimental conditions used for samples, and the so obtained blank curve was subtracted from those of samples to correct the error in the mass determination due to the reduction in the buoyancy force on increasing temperature. At the end of each experiment, TG data were used to plot the percentage of undegraded sample (1 − D) %, as a function of temperature, where D = (W o − W)/W o, and W o and W were the masses at the starting point and during scanning.
DSC measurements
A Mettler DSC 1 Star System was used for T g determinations. The calibration in enthalpy and temperature of the instrument was made following the procedure suggested by the manufacturer, which provide the fusion of indium for the enthalpy calibration and the melting points of indium and zinc, for that of temperature. The calibrations were repeated every 2 weeks. Samples of about 5.0 × 10−3 g, held in sealed aluminum crucibles, a heating rate of 10 °C min−1 were used for measurements. Multiple experiments were performed, and the considered values were averaged from those of three runs, the maximum difference between the average and the experimental values being within ±1 °C.
Results and discussion
The synthesized compounds were firstly spectroscopy characterized by 1 H NMR and FTIR in order to have a preliminary confirmation of the presence of POSSs in PS. FTIR spectra of nanocomposites were reported in Fig. 2 and compared with those of neat PS and POSS. A clear band attributable to Si–O bonds [11] was observed from 1094 to 1098 cm−1 for sample 1, 2 and 3, respectively. The shift of these bands with respect to that of POSS, observed at 1077 cm−1, seems to indicate the presence of filler–polymer interactions [16]. Moreover, the increase in the intensity of the band attributable to Si–O bond, passing from the spectrum of compound 1 to that of compound 3, was a qualitative confirmation of the different POSS percentages in the nanocomposites. The exact filler content was then calculated by the ratio of aromatic hydrogen atoms of POSS and those of PS, as described in experimental section through the spectra of the obtained compounds. The slightly higher POSS percentages, with respect to those in the reactant mixtures, that were found, namely 1.6 % (sample 1), 3.4 % (sample 2) and 5.2 % (sample 3), could be explained with the formation, in the reactant mixtures, of PS oligomers, which are soluble in methanol.
Thermal and thermal oxidative degradations of PS and nanocomposites were carried out in flowing nitrogen and in a static air atmosphere, respectively, with heating rates ranging from 2 to 20 °C min−1. The corresponding TG curves obtained at 10 °C min−1 in both atmospheres are presented in Figs. 3 and 4. Since the scanning rate largely affects both the shape and the position of TG curves, the thermal parameters of each sample at different scanning rates were different with each other, though the values of various samples at the same heating rate showed the same trend in every case. For the sake of simplicity, only the TG curves at a single scanning rate, namely at 10 °C min−1, were reported for comparison. This heating rate was selected because it is a medium rate among those we employed for degradation experiments and, also, that we used for glass transition temperature determinations. In all cases, degradation occurs through one sharp single stage and it is evident that nanocomposites degradation curves are shifted toward higher temperatures with respect to those of PS, thus indicating, at least qualitatively, higher resistance to thermal degradation. The temperature at 5 % mass loss was selected to evaluate thermal stability of our compounds. T 5 % was preferred because, differently than T i , it is not depending on the kinetics of the most advanced degradation stages [20]. Moreover, since all investigated compounds showed under both atmospheres, the same thermal behavior concerning the first TG step, which is the most important one to assess a thermal stability scale, the apparent activation energy of degradation, which is correlated with the kinetics of the process, was calculated.
The temperatures of DTG peaks at the various heating rates, in both flowing nitrogen (Table 1) and static air atmosphere (Table 2), which represent the temperatures at maximum rate of mass loss, were thus used to determine the degradation E a values of PS and nanocomposites through the classical Kissinger method [21], based on the following linear equation:
where Φ is the heating rate, T m is the temperature at maximum rate of mass loss, n is the apparent reaction order, R is the universal gas constant, A is the pre-exponential factor and W m is the mass of sample at the maximum rate of mass loss. Single linear ln (Φ/T 2m ) versus 1/T m relationships were obtained, at the various heating rates, for all samples in both studied environments. The corresponding regression coefficients and the calculated degradation E a values are reported in Tables 3 and 4 for nitrogen and air, respectively. To complete the thermal characterization, the glass transition temperatures of PS and nanocomposites were calorimetrically determined (Fig. 5) and reported in Table 5 together with T 5 % and degradation E a values.
The data in the Table 5 point out two important matters: while it is evident a higher overall thermal stability of hepta cyclopentyl bridged POSS/PS nanocomposites with respect to PS, it does not seem that there is a significant increase in thermal stability with increasing the percentage of POSS in the polymer matrix. Indeed, if a significant increase in both T 5 % (about 25 °C under nitrogen and 80 °C in static air atmosphere) and E a of degradation (about 30 and 40 kJ mol−1 under nitrogen and in static air respectively) was found passing from PS to various nanocomposites, the same cannot be observed passing from compound 1 to compound 3 for which only differences of about 10 °C and 10 kJ mol−1 were recorded. The enhancement of both T 5 % and degradation E a values observed for samples 1, 2 and 3 with respect to neat PS was slightly depending on the filler content. On considering that the effective modification of polymer matrices with POSSs depends, to a large extent, on the successful dispersion of the nanoparticles within the matrix, one can conclude that a good dispersion was obtained for these nanocomposites, but that this dispersion is limited by the POSS percentage in the matrix. Comparing the values obtained for the hepta cyclopentyl bridged POSS/PS nanocomposites with those filled with hepta isobutyl bridged ones previously studied [17], a considerable enhancement in the resistance to thermal degradation was observed. Since, at equal structure of the bridge connecting the two silicon cages, the vertex groups are expected to control POSS–POSS interactions and, thus, morphology and physical properties, this increase is probably due to the substitution of the isobutyl reactive groups with the cyclopentyl ones. Also the degradation activation energies of nanocomposites here studied were higher than those of nanocomposites filled with isobutyl bridged POSSs [19], in both investigated environments, thus meaning lower degradation rate and then better thermal stability from the kinetic viewpoint. Contrariwise, the T g values of compounds 1, 2 and 3 were lower than those found for the hepta isobutyl bridged POSSs [19]. Since the glass transition temperature reflects in the macroscale the mobility of component units of the material on the nanoscale, to explain this result we have to consider that the molecular dynamics of a polymer system are affected, to a large extent, by the network topology. POSSs cages chemically incorporated on the macromolecular chains can change the topology of the matrix and thus alter its dynamics [2]. The presence of reactive or less reactive POSSs during synthesis of the nanocomposite may affect the rate of the polymerization reactions, when the matrix is synthesized in situ, thus altering molecular mass and changing the degree of crosslinking and presumably influencing chain mobility. Since an high degree of crosslinking restricts the degrees of freedom for the chain as well as their mobility [22], the presence during polymerization of more reactive molecules (hepta isobutyl bridged POSSs), with respect to the less reactive ones (hepta cyclopentyl bridged POSSs), leads to highly crosslinked materials having higher T g.
Differently than PS that decomposed substantially with complete mass loss, comparable residue values among various nanocomposites were found, which were slightly higher for the degradations of compound 3, in agreement with higher POSS percentage in nanocomposite. The IR spectra of the solid residues of degradation of compound 3 (Fig. 6), obtained in both used atmospheres at 700 °C, showed exclusively bands associated with the presence of silica, and it is in agreement with literature data regarding the degradation of pure POSSs [23]. The absence of bands related to un-decomposed PS, or its decomposition products, seems to indicate the complete decomposition of polymeric matrix.
Conclusions
The here studied hepta cyclopentyl bridged POSS/PS nanocomposites, easily synthesized by in situ polymerization, exhibited a significant enhancement of their thermal stability with respect to those of pristine PS and of similar isobutyl bridged POSS/PS nanocomposites. The increase in both T 5 % and degradation E a values proved the influence of the nature of POSSs cage’s periphery on the thermal resistance and degradation rate of material. By contrast, the T g values indicated that the presence during in situ polymerization of less reactive nanoparticles (hepta cyclopentyl bridged POSSs), with respect to the more reactive ones (hepta isobutyl bridged POSSs), leads to materials with lower degree of crosslink.
References
Sanchez C, Julian-Lopez B, Belleville P, Popall M. Applications of hybrid organic–inorganic nanocomposites. J Mater Chem. 2005;15:3559–92.
Raftopoulos KN, Pielichowski K. Segmental dynamics in hybrid polymer/POSS nanomaterials. Prog Polym Sci. 2015;. doi:10.1016/j.progpolymsci.2015.01.003.
Wu Q, Zhang C, Liang R, Wang B. Combustion and thermal properties of epoxy/phenyltrisilanol polyhedral oligomeric silsesquioxane nanocomposites. J Therm Anal Calorim. 2010;100(3):1009–15.
Wang XT, Yang YK, Yang ZF, Zhou XP, Liao YG, Lv CC, Chang FC, Xie XL. Thermal properties and liquid crystallinity of side-chain azobenzene copolymer containing pendant polyhedral oligomeric silsequioxanes. J Therm Anal Calorim. 2010;102(2):739–44.
Devaraju S, Vengatesan MR, Selvi M, Alagar M. Thermal and dielectric properties of newly developed linear aliphatic-ether linked bismaleimide-polyhedral oligomeric silsesquioxane (POSS-AEBMI) nanocomposites. J Therm Anal Calorim. 2014;117(3):1047–63.
Villanueva M, Martín-Iglesias JL, Rodríguez-Añón JA, Proupín-Castiñeiras J. Thermal study of an epoxy system DGEBA (n = 0)/mXDA modified with POSS. J Therm Anal Calorim. 2009;96(2):575–82.
Lin PH, Khare R. Glass transition and structural properties of glycidyloxypropyl–heptaphenyl polyhedral oligomeric silsesquioxane-epoxy nanocomposites. J Therm Anal Calorim. 2010;102(2):461–7.
Zhang W, Li X, Yang R. Study on flame retardancy of TGDDM epoxy resins loaded with DOPO–POSS compound and OPS/DOPO mixture. Polym Degrad Stab. 2014;99:118–26.
Blanco I, Abate L, Bottino FA. Synthesis, characterization and thermal stability of new dumbbell-shaped isobutyl-substituted POSSs linked by aliphatic bridges. J Therm Anal Calorim. 2014;116:5–13.
Blanco I, Abate L, Bottino FA, Bottino P. Synthesis, characterization and thermal stability of new dumbbell-shaped isobutyl-substituted POSSs linked by aromatic bridges. J Therm Anal Calorim. 2014;117:243–50.
Blanco I, Abate L, Bottino FA. Synthesis and thermal characterization of new dumbbell-shaped cyclopentyl-substituted POSSs linked by aliphatic and aromatic bridges. J Therm Anal Calorim. 2015;121(3):1039–48.
Tang TTK, Zhao P, Yuen ACY, Yuen RKK, Lo SM. Hu Y. In: Kodur VKR, Banthia N, editors. Response of structures under extreme loading. Lancaster: DEStech Publications Inc.; 2015. p. 947.
Blanco I, Bottino FA. Thermal study on phenyl, hepta isobutyl-polyhedral oligomeric silsesquioxane/polystyrene nanocomposites. Polym Compos. 2013;34(2):225–32.
Blanco I, Abate L, Bottino FA, Bottino P. Phenyl hepta cyclopentyl-polyhedral oligomeric silsesquioxane (ph, hcp-POSS)/Polystyrene (PS) nanocomposites: the influence of substituents in the phenyl group on the thermal stability. Express Polym Lett. 2012;6(12):997–1006.
Blanco I, Abate L, Bottino FA. Variously substituted phenyl hepta cyclopentyl-polyhedral oligomeric silsesquioxane (ph, hcp-POSS)/polystyrene (PS) nanocomposites. J Therm Anal Calorim. 2013;112(1):421–8.
Blanco I, Bottino FA, Bottino P. Influence of symmetry/asymmetry of the nanoparticles structure on the thermal stability of polyhedral oligomeric silsesquioxane/polystyrene nanocomposites. Polym Compos. 2012;33(11):1903–10.
Blanco I, Abate L, Bottino FA, Bottino P. Thermal behaviour of a series of novel aliphatic bridged polyhedral oligomeric silsesquioxanes (POSSs)/polystyrene (PS) nanocomposites: the influence of the bridge length on the resistance to thermal degradation. Polym Degrad Stab. 2014;102:132–7.
Blanco I, Abate L, Bottino FA, Cicala G, Latteri A. Dumbbell-shaped polyhedral oligomeric silsesquioxanes/polystyrene nanocomposites: the influence of the bridge rigidity on the resistance to thermal degradation. J Compos Mater. 2015;49(20):2509–17.
Blanco I, Bottino FA, Cicala G, Latteri A, Recca A. A kinetic study of the thermal and thermal oxidative degradations of new bridged POSS/PS nanocomposites. Polym Degrad Stab. 2013;98(12):2564–70.
Abate L, Blanco I, Cicala G, Recca G, Scamporrino A. The influence of chain rigidity on the thermal properties of some novel random copolyethersulfones. Polym Degrad Stab. 2010;95(5):798–802.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Abate L, Blanco I, Bottino FA, Di Pasquale G, Fabbri E, Orestano A, Pollicino A. Kinetic study of the thermal degradation of PS/MMT nanocomposites prepared with imidazolium surfactants. J Therm Anal Calorim. 2008;91(3):681–6.
Fina A, Tabuani D, Carniato F, Frache A, Boccaleri E, Camino G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim Acta. 2006;440(1):36–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blanco, I., Bottino, F.A. Thermal characterization of a series of novel hepta cyclopentyl bridged POSS/PS nanocomposites. J Therm Anal Calorim 125, 637–643 (2016). https://doi.org/10.1007/s10973-015-5098-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5098-7