Abstract
The comprehension of the thermal phenomena of milk powders is still object of investigation, being the result of complex interactions among their components as affected by composition, storage conditions and manufacturing. For a better understanding of the above-mentioned phenomena, the effect of the single milk component (proteins, casein, whey proteins and lactose) on the thermal behavior of whole and skim milk powders was investigated by means of DSC during heating. Whole milk powders exhibited two endothermic transitions related to the fat crystal melting at temperatures between 276 and 325 K. Glass transitions of lactose (309 K) and caseins (347–358 K) with their enthalpy relaxation phenomena were observed in the milk derivatives, being instead highly influenced from a w and composition in the dried milk. At higher temperature (353–473 K), different exothermic events, such as lactose crystallization, Maillard reaction and/or decomposition of carbohydrates and proteins, were recognized in the curves of milk and protein derivatives powders. A good explanation of the thermal properties of the milk powders was obtained from the comparison with the heating curves of the single components. Lactose resulted the key component of many thermal events, being the molecule more influenced by the environmental conditions. These results can lead to a better comprehension of the physical properties of the milk powders, fundamental for their functional and commercial quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milk powder manufacture is a process that involves the removal of water from the concentrated milk mainly by spray drying into a flow of hot air with low percentage relative humidity (RH %). Dried milk is a convenient and easy to use dairy product, because of low-cost transport and long-term storage at room temperature. According to the drying conditions, the main milk components might be involved in various modifications, e.g., chemical modifications (Maillard reaction, protein denaturation, protein to protein aggregation, protein to fat interactions and protein to carbohydrate bonds) or physical ones (lactose crystallization, stickiness, collapse and aroma retention) [1, 2]. The nature and the extent of these modifications may depend on many variables, such as composition, processing and storage conditions [3–6].
The use of the differential scanning calorimetry (DSC) for the assessment of the food quality changes during processing and storage has been already successfully proposed [7, 8]. It was also considered as a powerful tool to record physical changes occurring during milk dehydration by spray drying and during storage [9–11].
The application of this technique to the field of milk powders, however, focuses mostly on the study of the thermal behavior of the milk components. Among these, lactose, the most abundant component of dried milk, is involved in the major thermal reactions whether it is in amorphous or crystalline form, characterized by different thermal properties [12–14], and the crystallization of amorphous form in dairy powders during storage is one of the main causes of quality loss [15, 16]. If not crystallized within the process, the amorphous lactose [17] can convert from a glassy-to-rubbery state when stored above its glass transition temperature (T g) and according to the relative humidity [5, 15, 18, 19]; the increase in molecular mobility and the decrease in viscosity may cause stickiness, caking and crystallization [17]. The presence of the other components such as proteins and fats can largely affect the physicochemical properties of all milk powders, including water absorption, glass transition and crystallization [14]. According to Jouppila et al. [20], proteins seem to limit and delay crystallization of lactose in milk powders compared with pure lactose. In addition, the denaturation of proteins [11, 21, 22] and their aggregation [2] originate endothermic and exothermic peaks, respectively, well detected by means of DSC. Finally, milk fat is characterized by a great number of triglycerides, originating different melting temperatures that could be observed by DSC in a broad endothermic peak range, varying between 233 and 313 K [23, 24].
While the association of each transition to a molecular modification may be easier when mono or two-component systems are tested, the explanation of a multi-component matrix as dried milk is more complex and not unequivocal because of the interactions between the components and/or the superposition of phase transitions [25]. Few studies are available on the thermal relationship existing between milk powders and their components [9, 14, 17, 18, 20, 22, 25], and for most of them, the heating scan stopped at temperature lower than 453 K.
Thus, starting from the statement that DSC peaks of dried milk can be different from those of raw milk, because of the interactions of the milk components as modified by the process, the actual objective of this work was to study for the first time in the literature the heating behavior up to 498.15 K of several samples of whole and skim dried milk, in comparison with commercial dried dairy powders (casein, whey protein concentrates, lactose) resulting from the milk cracking and characterized by a large prevalence of one or two components.
Materials and methods
Samples
The dried milk samples used in this study were three spray-dried whole milk (WM1–3), one whole milk (WM4) obtained by roller drying method, eleven spray-dried skimmed milk (SM1–11) and dried milk derivatives such as lactose (LAT), milk protein concentrate (MPC), presamic casein (CAP), acid casein (CAA), sodium caseinate (CAS), whey protein concentrate (WPC) and whey protein isolate (WPI). The commercial products were collected from several European manufacturers (Spain, Germany, Ireland and Poland). Compositional data were given by manufacturers. SM powders had <1.5 % of fats, lactose in the range of 51.5–56.0 % and proteins and ash content in the range of and 33.1–36.5 % and 6.2–9.5, respectively. The WM powders had 26.0 % of fats approximately, lactose in the range 36.8–40.0 %, proteins and ash from 24.0 to 26.2 % and 5.5–7.0, respectively. Lactose was pure at 99 %, while the protein content of MPCs, CAP, CAA, CAS, WPC and WPI was 82, 91, 95, 90.5, 79.3, 92 %, respectively. Moisture was usually <4.5 %, with the exception of casein products where it was <12 %.
Water activity
Water activity (a w) of all the samples was measured at 298.15 K using a Decagon Aqualab Meter Series 3TE (Pullman, WA, USA) and determined as the mean of three measurements.
DSC analysis
Samples (8–10 mg) were weighed into aluminum pans, accurately weighted with an analytical scale (Ohaus AR2140, Florham Park, NJ, USA), and covers were sealed into place and analyzed with a DSC Q100 (TA Instruments, New Castle, DE, USA). Indium (melting temperature 429.75 K, ΔHf = 28.45 J g−1) and mercury (melting temperature 234.35 K, ΔHf = 11.4 J g−1) were used to calibrate the instrument, and an empty pan was used as reference. Pans with samples were equilibrated at 298.15 K for 3 min and then cooled at 193.15 K at the rate of 5 K min−1, equilibrated at 193.15 K for 5 min and then heated from 193.15 K to 498.15 at 5 K min−1. Dry nitrogen was purged in the DSC cell at 50 mL min−1. The curves were analyzed with Universal Analysis Software (version 3.9A, TA Instruments) to obtain peak temperature at the beginning, Tonset (T on), and at the maximum, Tpeak (T p), of the transition. Glass transition temperature (T g) was recorded as the T on of the corresponding transition.
Three replicates were analyzed per sample, and data were tabulated as average value ± standard deviation.
Results and discussion
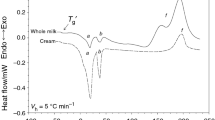
The heating curves of all the dried milk and milk derivatives samples are shown in Figs. 1–5. Detected peaks are indicated with a different letter (from a to l).
Multiple thermal events are shown over the entire range of temperature. In particular, the curves of the dried milk samples are quite similar among them despite their different origins and probable manufacturing process conditions. On the contrary, milk derivatives curves presented specific behaviors.
Several differences were, however, found, and curves will be described focusing on the single thermal event starting from the lowest to the highest temperature. Due to the complexity of the curve profiles, characterized by successions and overlapping of endothermic, exothermic and glass transitions, the tracking of the baseline could not be accurate. The enthalpy calculation was thus not considered for describing the observed transitions.
To favor the discussion about the possible meaning of the peaks found in the curves of each sample, the temperatures (T on and T p) of the major transitions are reported in Tables 1 and 2, together with the a w values.
Endothermic transitions
Whole milk powders exhibited two endothermic transitions (peaks a and b) related to the fat crystal melting in the range of temperature from 276.2 to about 320 K (Fig. 1); T p values of these peaks were about 291 K for the first peak and from 307.2 to 316.2 K for the second peak (Table 1). In all the skim milk powder samples, these peaks did not appear. Different numbers and/or maximum temperatures were shown in the literature for these peaks in relation to the composition of the matrix [2, 25]. Grotenhuis et al. [24], analyzing anhydrous milk fat, observed three endothermic peaks, the first in the range from 233 to 281 K, the second relatively sharp and ended at 293 K, while the third was found at approximately 308 K. However, it should be noted that the presence of the first peak was related to the rate of cooling before the melting step. Rahman et al. [25], analyzing camel milk, found two peaks related to the fat melting with T on and T p at 283 and 290 K for the first peak and at 307 and 314 K for the second peak, respectively. Peak temperatures resulted different for pure camel fat and cream, with the first peak at lower and the second at higher temperature. Aguedo et al. [26] found only one peak at 283.4 K for milk fat applying a melting rate of 15 K min−1, while Ostrowska-Ligeza et al. [22] showed two peaks at 345.1 and 326 K analyzing whole milk powder.
Denaturation of whey proteins (α-lactalbumin, β-lactoglobulin) may occur in the temperature region of 323–363 K depending on the powder property and process adopted for the production [2, 21, 27]. Both dried whole and skim milk powders did not show endothermic peaks in this region, probably because the drying process already led to a denaturation of the whey proteins and/or their interactions with casein. The overlapping of the denaturation peak with other transitions cannot be excluded, too. On the other hand, the analysis of the commercial whey protein concentrates (WPI and WPC, Fig. 5) confirmed the data reported in the literature [2, 21, 27], as these curves showed an endothermic transition at the T p of 347 and 346.4 for WPI and WPC, respectively.
Casein micelles, the major milk protein component, lack tertiary globular structure during heating; thus, pure casein should not show endothermic transitions under DSC experiments [28, 29]. In this study, however, where commercial caseins samples (CAS, CAP, CAA) have been heated in the region between 330 and 350 K (Fig. 5), an endothermic transition (m*) immediately before a glass transition (m) was visible, more evident in samples CAP and CAA than CAS. Similarly, Bengoechea et al. [30] found an endothermic peak immediately before the glass transition temperature on rennet caseins. These authors hypothesize that the endothermic transition could be associated with the proteins’ physical aging (enthalpy relaxation) phenomena.
The heating curves of the milk protein concentrates (MPC, Fig. 5) showed a behavior similar to that of caseins. MPC is a mixture of concentrated milk proteins, and thus, the shape of its curves corresponds to the reaction of the major component. Moreover, simultaneous unfolding (endothermic) reactions of the whey proteins and interactions (exothermic) between the unfolded globular whey proteins and caseins can be superimposed in the same temperature range [31].
As reported above, the bigger complexity of the DSC profiles of skim and whole milk powders compared to protein concentrates did not allow to detect these transitions in dried milk powder curves, probably due to the overlapping with the T g/enthalpic relaxation transition and with other exothermic transitions. It is also reported [32] that in mixed system the glass transition may be broad, making difficult the DSC detection. Moreover, because of the known plasticizing effect of sugars on proteins, lactose may have caused the decrease in the protein glass transition and the overlapping with certain other transitions visible at lower temperatures [32].
Glass transition
In the present study, dried milk samples were analyzed as they arrived to the laboratory and without further modifications. The water content (according to the producer data) and the water activity (Tables 1, 2) of the samples were in the range 2.4–5.0 % and 0.144–0.329, respectively. A glass transition was observed in all the dried milk samples, with the exception of WM2, in the temperature range from about 315–330 K (Table 1; Figs. 1, 2, 3, peak c).
It is well known that the presence of the glass transition (T g) and its variability are strongly related to the physical state of the powder given by the drying process, as well as the chemical composition and the water activity (a w) [33]. Fernandez et al. [18] measured T g values of 334 and 335 K for whole and skim milk, respectively.
Ozmen et al. [34] found decreasing T g values of skim milk powder in the range from 360.8 to 319.8 K, with a water content increasing from 1.65 to 4.52 %. Hogan et al. [35] found a similar trend of T g values for skim milk powders; its temperatures decreased from 334.1 to 284.1 K as moisture content and a w increased from 1.9 to 8.1 % and from 0.10 to 0.38, respectively.
Jouppila et al. [20] affirmed that T g could not be determined in whole milk powders with moisture content higher than 4.3 % due to the overlapping of fat melting endotherm and T g in the same temperature range. In samples of skim and fat containing milk, with water content lower than 4.3 %, T g was found at temperature between 331.1 and 336.1 K [20]. Rahman et al. [25] measured a T g value of 304.1 K for whole camel milk, with a moisture fraction of 7.9 %.
In this study, the dried milk samples (Figs. 1–3, peak c) and the amorphous lactose (Fig. 4, peak g) showed typical glass transitions. For lactose, the onset T g value of 309.2 K (Fig. 4, peak g), at a a w lower than 0.05 (Table 2), was found. Haque et al. found a higher T g value of 365.1 K for anhydrous lactose [36]. However, a w hardly affects onset T g value, as shown by Jouppila and Roos [20], who found that the glass transition of amorphous lactose occurs at 297.15 K at a water content of about 6.8 %.
Focusing on the dried whole and skim milks, the T g (Table 1) visible in the range 315–332 K showed an high inverse correlation (Pearson correlation coefficient R = −0.77; p < 0.01) with a w, confirming the plasticizing effect of water on the glass transition.
As previously explained, protein milk derivative curves (MPC, CAS, CAP and CAA), except whey proteins, exhibited a glass transition (peak m, Fig. 5; Table 2) after the endothermic enthalpic relaxation (peak m*, Fig. 5; Table 2), in the range of temperature from 341.9 to 349.5 K. Rahman et al. [25] observed a glass transition of casein and whey proteins at onset temperature of 350.1 and 368.1 K, respectively.
Exothermic peaks related to milk components modifications and/or interactions
An enthalpy relaxation phenomenon, typically associated with the peaks of glass transitions (Figs. 1–3, peak c; Fig. 4, peak g), is observed in milk powders and in lactose [9]. Enthalpy relaxations may be either endothermic or exothermic, depending on the thermal history of the material and the timescale of observation [33]. In the conditions used in this study, an exothermic peak (Figs. 1–3, peak c*; Fig. 4, peak g*) was visible after the lactose glass transition (Figs. 1–3, peak c; Fig. 4, peak g). The exothermic phenomenon was associated with an enthalpic relaxation, representative of a certain degree of mobility of the molecules [35, 37–39].
In Figs. 1–3, one (WM1 and SM6 to SM 8 samples) or two (all the other samples) exothermic transitions (peaks d and e), followed by a large exothermic peak preceded by a not defined shoulder (peak f), were observed. The T on of these peaks was measured at temperatures ranging between 356.8 and 393.2 K for the first peak (Table 1, peak d) and 389.1 and 424.4 K for the second peak (Table 1, peak e). It is possible to hypothesize that these transitions were not the consequence of a single isolated reaction, but the results of different overlapped thermal events, such as lactose crystallization, Maillard reaction and/or decomposition or denaturation of carbohydrates and proteins. Furthermore, a small and flat transition was also visible at 292.4 K in WPC (Table 2, peak j).
The transitions reported in Fig. 5 above 373 K were difficult to interpret and detect due to the minor drifts of the baseline. MPCs and WPC powders exhibited a transition with T p in the range between 402.3 and 414.2 K (Table 2, peak l). According to Morgan et al. [1], lactose crystallization and Maillard reaction are the two major modifications occurring in milk and whey powders during processing and storage. It is well known that milk proteins and other components delay the peak [40] and the rate [20] of lactose crystallization in milk powder. In the present study, amorphous lactose (obtained by lyophilization) showed an exothermic transition with T on and T p at 347.6 and 359.9 K, respectively (Fig. 4 and Table 2, peak h), attributable to the crystallization phenomenon, according to the literature results. Haque et al. [40] and Morgan et al. [1] reported a crystallization temperature of pure lactose ranging from 340.3 to 390.5 K, and from 335.1 to 363.1 K, respectively, in a range of activity water similar to the ones found in the present study. However, crystallization temperature of pure lactose is lower than those of lactose in milk powders. Generally, T on decreased as water content increased [20], and the presence of fat and proteins retards lactose crystallization [20, 41]. Moreover, it is well known that the samples composition and the relative processing methods impact on the temperature of the phase transitions resulting in more or less marked shift of the peaks.
The last large transition of milk powders heating curves (Figs. 1–3, peak f) may be s instead accountable to the decomposition of lactose (465.2–469.6 K). In the heating curve of the amorphous lactose, an exothermic transition beginning at the temperature of 498.7 K was found (Fig. 4) (data not reported in Table 2). Ostrowska-Ligęza et al. [22] found a similar transition in milk powder samples in the range 473.1–544.1 K, and they attributed this peak to the sample decomposition. On the other hand, Rahman et al. [25] described as “structure forming” the exothermic transition found in dried camel milk around 473 K.
Conclusions
The findings of this study add new literature information about thermal behavior of milk powders and the effect of components on DSC curves. DSC analysis of commercial dried whole and skim milk did not show relevant differences among the analyzed samples, although some of them had a specific thermal profile, probably caused by unknown manufacture processing conditions and/or storage. However, the comparison of the DSC curves of milk powder samples with those of commercial dried milk derivatives (proteins with or without lactose and pure lactose) confirmed the crucial role of lactose in the thermal behavior of milk powder. Modifications of lactose, caused by the processing, are clearly shown by the exothermic peaks above 430 K, while the presence of a glass transition followed by an exothermic enthalpic recovery makes possible to outpoint the presence of amorphous lactose in the sample. Knowing the lactose behavior in dried milk can help to prevent the crystallization of amorphous lactose controlling the water migration during storage at temperature below the T g value.
The absence, in the milk powders, of endothermic peaks usually found in WPC and associated with whey protein heat denaturation, is probably due to the whey proteins complete denaturation during drying, or to possible interactions with casein. Moreover, this interaction can contribute to modifying the glass transition of casein.
The findings of this study revealed that a deeper understanding of DSC curves of dried milk may result in the future possibility to apply thermal analysis to discriminate dried milk characteristics not only on the basis of their components, but also on the influence of drying process and/or storage on composition. This aspect deserves to be deepened with further studies on the matter.
References
Morgan F, Nouzille Appolonia C, Baechler R, Vuataz G, Raemy A. Lactose crystallisation and early Maillard reaction in skim milk powder and whey protein concentrates. Lait. 2005;85:315–23.
Fitzsimons SM, Mulvihill DM, Morris ER. Denaturation and aggregation processes in thermal gelation of whey proteins resolved by differential scanning calorimetry. Food Hydrocoll. 2007;21:638–44.
Tsourouflis S, Flink JM, Karel M. Loss of structure in freeze-dried carbohydrates solutions: effect of temperature, moisture content and composition. J Sci Food Agric. 1976;27:509–19.
To EC, Flink JM. “Collapse”, a structural transition in freeze-dried carbohydrates. III Prerequisite of recrystallization. J Food Technol. 1978;1:586–94.
White GW, Cakebread SH. The glassy state in certain sugar-containing food products. J Food Technol. 1966;1:73–82.
Lazar ME, Brown AH, Smith GS, Wong FF, Lindquist FE. Experimental production of tomato powder by spray drying. Food Technol. 1956;10:129–34.
Roos YH. Thermal analysis, state transitions and food quality. J Therm Anal Calorim. 2003;71:197–203.
Raemy A. Behavior of foods studied by thermal analysis. J Therm Anal Calorim. 2003;71:273–8.
Vuataz G. The phase diagram of milk: a new tool for optimising the drying process. Lait. 2002;82:485–500.
Roos YH. Importance of glass transition and water activity to spray drying and stability of dairy powders. Lait. 2002;82:475–84.
Thomas M, Scher J, Desobry S. Study of lactose/α-lactoglobulin interactions during storage. Lait. 2005;85:325–33.
Chan HK, Chew NYK. Novel alternative methods for the delivery of drugs for the treatment of asthma. Adv Drug Deliv Rev. 2003;55:793–805.
Kim EHJ. Surface composition of industrial spray-dried dairy powders and its formation mechanisms. Ph.D. Thesis, University of Auckland, Auckland; 2008.
Shrestha AK, Howes T, Adhikari BP, Bhandari BR. Water sorption and glass transition properties of spray dried lactose hydrolysed skim milk powder. LWT Food Sci Technol. 2007;40:1593–15600.
Supplee GC. Humidity equilibria of milk powders. J Dairy Sci. 1926;9:50.
Troy HC, Sharp PF. α and β lactose in some milk products. J Dairy Sci. 1930;13:140–57.
Vuataz G. Preservation of skim-milk powders: role of water activity and temperature in lactose crystallization and lysine loss. In: Seow CC, editor. Food preservation by water activity control. Amsterdam: Elsevier; 1988. p. 73–101.
Fernández E, Schebor C, Chirife J. Glass transition temperature of regular and lactose hydrolyzed milk powders. LWT Food Sci Technol. 2003;36:547–51.
Slade L, Levine H. Beyond water activity: recent advances based on an alternative approach to the assessment of food quality and safety. Crit Rev Food Sci Nutr. 1991;30:115–360.
Jouppila K, Roos YH. Glass transitions and crystallization in milk powders. J Dairy Sci. 1994;77:2907–15.
Boye JI, Alli I. Thermal denaturation of mixtures of alpha-lactalbumin and beta-lactoglobulin: a differential scanning calorimetric study. Food Res Int. 2000;33:673–82.
Ostrowska-Ligęza E, Górska A, Wirkowska M, Koczoń P. An assessment of various powdered baby formulas by conventional methods (DSC) or FT-IR spectroscopy. J Therm Anal Calorim. 2012;110:465–71.
Kim EHJ, Chen XD, Pearce D. Melting characteristics of fat present on the surface of industrial spray-dried dairy powders. Colloids Surf B Biointerfaces. 2005;42:1–8.
Grotenhuis E, Van Aken GA, Van Malssen KF, Schenk H. Polymorphism of milk fat studied by differential scanning calorimetry and real-time X-ray powder diffraction. J Am Oil Chem Soc. 1999;76:1031–9.
Rahman MS, Al-Hakmani H, Al-Alawi A, Al-Marhubi I. Thermal characteristics of freeze-dried camel milk and its major components. Thermochim Acta. 2012;549:116–23.
Aguedo M, Giet JM, Hanon E, Lognay G, Wathelet B, Destain K, Brasseur R, Vandenbol M, Danthine S, Blecker C, Wathelet JP. Calorimetric study of milk fat/rapeseed oil blends and their interesterification products. Eur J Lipid Sci Technol. 2009;111:376–85.
De Wit J, Klarenbeek G. Effects of various heat treatments on structure and solubility of whey proteins. J Dairy Sci. 1984;67:2701–10.
Paulson M, Dejmek P. Thermal denaturation of whey proteins in mixtures with caseins studied by differential scanning calorimetry. J Dairy Sci. 1999;73:590–600.
Erdogdu N, Czuchajowska Z, Pomeranz Y. Wheat-flour and defatted milk fractions characterized by differential scanning calorimetry. I. DSC of flour and milk fractions. Cereal Chem. 1995;72:70–5.
Bengoechea C, Arrachid A, Guerrero A, Hill SE, Mitchell JR. Relationship between the glass transition temperature and the melt flow behavior for gluten, casein and soya. J Cereal Sci. 2007;45:275–84.
Relkin P. Heat-induced phase transformations of protein solutions and fat droplets in oil-in-water emulsions: a thermodynamic and kinetic study. In: Kaletunç G, editor. Calorimetry in food processing: analysis and design of food systems. Ames: Wiley-Blackwell and IFT Press; 2009. p. 119–45.
Kalichevsky MT, Blanshard JMV, Tokarczuk PF. Effect of water content and sugars on the glass transition of casein and sodium caseinate. Int J Food Sci Technol. 1993;28:139–51.
Roos YH. Water activity and physical state effects on amorphous food stability. J Food Process Preserv. 1993;16:433–47.
Ozmen L, Langrish TAG. Comparison of glass transition temperature and sticky point temperature for skim milk powder. Dry Technol. 2002;20:1177–92.
Hogan SA, Famelart MH, O’Callaghan DJ, Schuck P. A novel technique for determining glass–rubber transition in dairy powders. J Food Eng. 2010;99:76–82.
Haque MK. Glass transition and enthalpy relaxation of amorphous food saccharides: a review. J Agric Food Chem. 2006;54:5701–17.
Chung HJ, Lim ST. Physical aging of amorphous starches (a review). Starch. 2006;58:599–610.
Sartor G, Mayer E, Johari GP. Calorimetric studies of the kinetic unfreezing of molecular motions in hydrated lysozyme, hemoglobin, and myoglobin. Biophys J. 1994;66:249–58.
Haque E, Whittaker AK, Gidley MJ, Deeth HC, Fibrianto K, Bhandari BR. Kinetics of enthalpy relaxation of milk protein concentrate powder upon ageing and its effect on solubility. Food Chem. 2012;134:1368–73.
Haque MK, Roos YH. Water plasticization and crystallization of lactose in spray-dried lactose/protein mixtures. J Food Sci. 2004;69:23–9.
Karmas R, Buera MP, Brunswick N. Effect of glass transition on rates of nonenzymatic browning in food systems. J Agric Food Chem. 1992;40:873–9.
Acknowledgements
The research leading to these results has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration within the SUSMILK project (www.susmilk.com), Grant Agreement No. 613589.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pugliese, A., Paciulli, M., Chiavaro, E. et al. Characterization of commercial dried milk and some of its derivatives by differential scanning calorimetry. J Therm Anal Calorim 123, 2583–2590 (2016). https://doi.org/10.1007/s10973-016-5243-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5243-y