Abstract
Ternesite is an intermediate phase formed during clinkering of calcium sulfoaluminate (CSA) cement. This paper presents an experimental study on the feasibility of using ternesite as an additive of CSA cement in order to improve cement properties. Properties including setting time, mechanical strength, dimensional stability and hydration kinetics are investigated. The results indicated that the incorporation of ternesite can decrease the setting times and the strength development at early ages. The hydration of ternesite at later ages can increase the strength after 28 days of hydration, and 5% of ternesite is most favorable. The use of ternesite as an additive in CSA cement is harmless to the volume stability. The addition of ye’elimite to the pure ternesite promotes the hydration of ternesite and the formation of ettringite. XRD results show that the addition of ternesite can promote the formation of ettringite at later ages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to increasing pressure to reduce CO2 emissions during cement production, industries worldwide are focusing on alternative approaches for the production of more environmental friendly cement. A promising low-CO2 alternative is the production of calcium sulfoaluminate (CSA) cement. A typical CSA cement consists of predominantly ye’elimite (calcium sulfoaluminate), belite and smaller amount of aluminoferrite. CSA cement is obtained by burning raw materials like limestone, bauxite and gypsum within a temperature range of 1250–1350 °C. This is 100 up to 200 °C lower than Portland cement (PC) production, and thus, the energy input requirement is lower [1]. Compared to alite and belite, calcium sulfoaluminate contains lower CaO, which results in a clear reduction in CO2 emissions during the calcinations of limestone in cement kilns. In contrast to Portland cement, CSA cement exhibits superior features including high early strength, rapid setting, shrinkage compensating and self-stressing, which are attributed to the fast hydration rate of ye’elimite and the ettringite formation [2]. The main uses of CSA cement are for quick repairs and precast products [3].
Ternesite (\({\text{C5S2}}\overline{\text{S}}\)) is an intermediate phase formed during the burning process of CSA cement clinkers. Ternesite is a calcium sulfosilicate, formed in the reaction between belite and anhydrite. The formation of ternesite is strongly affected by firing temperature. Previous investigations on systems containing CaO, SiO2, Al2O3 and CaSO4 found that ternesite is stable in the temperature range between 900 and 1200 °C [4, 5]. Hou et al. showed that the formation temperature of ternesite is in the range 1100–1200 °C and it will decompose to belite and anhydrite when the temperature is higher than 1200 °C [6]. Wang et al. studied the high-temperature reactions during clinkering of CSA cement clinkers [7]. The results showed that at 1150–1250 °C, belite reacts with anhydrite to form ternesite, which decomposes to the above two components at temperatures above 1250 °C. Therefore, ternesite does not form in traditional CSA cement clinkers. Additionally, the formation of ternesite was observed to be linked to the SO3-to-(Al2O3 + Fe2O3) ratio [8]. The increase in the SO3 content in the raw meal leads to the replacement of large amounts of C2S by ternesite at temperatures of below 1250 °C.
Ternesite has traditionally been regarded as hydraulically inactive and of no technological interest [9, 10]. Sherman et al. showed that the reactivity degree of pure ternesite at 7 days and 2 years is only 3% and 12%, respectively [9]. Therefore, ternesite was generally identified as unwanted phase in CSA cement clinkers. In contrast, recent studies have shown that ternesite is a reactive phase when part of belite calcium sulfoaluminate ferrite clinkers and it is more reactive than belite [8]. The work of Shen et al. [11] has focused on the production of belite sulfoaluminate-ternesite clinker based on two successive firing steps. They found that the presence of large amount of ternesite can increase the strength after 56 days of hardening. The results also showed that the ettringite formation for the clinker containing ternesite mainly depends on the dissolution of gypsum originating from the hydration of ternesite. The hydraulic reactivity of ternesite can be ascribed to the aluminum released during the hydration of ye’elimite [12]. In the presence of reactive aluminum sources (calcium sulfoaluminate), the pore solution is undersaturated with respect to Ca, S and Si ions, and thus, the undersaturation promotes the dissolution of ternesite [8]. It was found that the dissolution of ternesite is accompanied with the release of a highly reactive calcium silicate phase that consumes Al(OH)3 to form strätlingite. Further dissolution of ternesite increases the calcium and sulfate concentration followed by Al(OH)−4 consumption, and ettringite starts to precipitate [12]. Consequently, these hydration products actively contribute to the mechanical strength and durability.
This work aims at giving new insight into the influence of ternesite on the hydration kinetics and mechanical properties of CSA cement. Additionally, the effect of calcium sulfoaluminate on the hydration behavior of the synthetic ternesite was also discussed to better understand the activation of calcium sulfoaluminate on the reactivity of ternesite.
Experimental
Raw materials
The commercial CSA cement, industrially produced in Hebei Province of China, was used in this research. The chemical composition of CSA cement is given in Table 1. The main mineralogical compositions determined by the quantitative XRD Rietveld analysis were ye’elimite (43.2 mass %), belite (36.6 mass %), anhydrite (16.0 mass %) and calcite (4.2 mass %). The specific surface are of CSA cement was 370 m2 kg−1. The reagent grade chemicals used for the synthesis of pure ternesite were calcium carbonate and calcium sulfate dihydrate. Silica fume was used as silicon provider, and its chemical composition is also listed in Table 1. It shows that silica fume consists primarily of silicon dioxide. The surface area of silica fume was 21, 000 m2 kg−1. The high silica content and large surface area of silica fume are beneficial to the synthesis of pure ternesite.
Synthesis of pure ternesite
Pure ternesite was synthesized from stoichiometric amounts of calcium carbonate, calcium sulfate dihydrate and silica fume. These materials were homogenized and then pelletized to the size of approximately 20-mm diameter with 10% of water. The pellets were dried at 100 °C for 24 h and then fired in porcelain crucibles in a Si–Mo rod resistance furnace according to the protocols shown in Fig. 1. First, the pellets were fired at 1150 °C for 4 h after ramping up at a heating rate of 5 °C min−1 and then removed from the furnace and rapidly cooled with forced air. The samples were ground into fine powders and pressed into pellets again. A second firing cycle, which is the same as the first firing, was carried out to enhance the formation of ternesite and followed by forced air cooling. The samples so prepared were ball milled to a Blaine fineness of about 320 m2 kg−1.
Testing methods
For the study of ternesite as an additive in CSA cement, combinations were prepared at varying ternesite contents, i.e., CSA/ternesite: 100/0, 95/5, 90/10 and 85/15. The setting times of the pastes were determined according to Chinese standard GB/T 1346-2001. The w/c ratio used for setting time was 0.3. Standard mortars were prepared with water/cement/sand ratio of 0.5/1/3 and mechanically homogenized according to Chinese standard GB/T 17,671-1999. Prismatic samples (40 mm × 40 mm × 160 mm) were cast and cured at 20 ± 1 °C and 95% relative humidity during 24 h. Then, the samples were demolded and cured in water at 20 ± 1 °C until measurements were performed. Compressive strengths were measured at 3, 7, 28, 56 and 90 days, and the reported values are the average of six samples. Dimensional stability was tested by measuring length changes of 25 mm × 25 mm × 280 mm mortar prisms. The composition of the mortars was the same as that of prismatic mortars. After 24 h of moist curing at 20 ± 1 °C, the mortar bars were demolded, and the initial lengths were taken. The specimens were subsequently cured in air and water, respectively, to the desired testing age for the measurement of the shrinkage/expansion ratio.
In order to characterize the hydration process, the hydration heat release of pastes was monitored by means of isothermal heat conduction calorimetry using a TAM air calorimeter. The heat flow was collected within the first 25 h at a temperature of 20 °C and an atmosphere of air. The cement pastes (water/cement ratio of 0.5) were mixed before testing.
Cement pastes for studies of hydration products were prepared with a constant water-to-cement ratio (w/c) of 0.4. The hydration samples were collected from the middle sections of the paste specimens. The samples at different curing ages were stopped by immerging into absolute alcohol for 48 h. Finally, the samples were dried in a vacuum chamber and ground to pass through a 80-μm sieve prior to analysis.
The mineral phases were identified using the X-ray diffraction (XRD) method. The XRD patterns were recorded on a Bruker D8 Advance X-ray diffractometer with Cu Kα radiation. The X-ray diffraction instrument was operated under 40 kV and 40 mA. The quantitative analysis of minerals was conducted using Rietveld refinement performed with TOPAS 4.2 software. Scanning electron microscopy (SEM) was used to examine the microstructure and morphology of hydration products. A gold coating was applied onto the surface of the samples to promote electrical conductivity.
Results and discussion
Synthesis of ternesite
The XRD pattern of the synthetic ternesite is shown in Fig. 2. The characteristic peaks of ternesite are clearly identified, while belite and anhydrite are observed as minor phases. Free lime is not detected, revealing that the 1150 °C firing temperature is sufficient for mineral formation. Ternesite is an intermediate phase generally present at temperatures between 1100 and 1250 °C during the crystallization of belite in CSA cements [8, 13, 14]. The result of Rietveld analysis shows that the ternesite content in the final product reaches approximately 92%, which is higher than that synthesized at 1200 °C for 8 h by Shen et al. [11]. As a result, applying the second firing process is more effective in the preparation of high-purity ternesite. Although small amounts of belite and anhydrite are found, their presence can be negligible when the synthesized ternesite is added as an additive in CSA cements.
Heat development
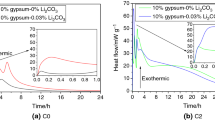
The heat evolution of CSA cements with increasing amounts of ternesite contents is illustrated in Fig. 3. The presence of ternesite results in different heat flow at a given time. The heat release during the initial period is mainly assigned to the wetting of the system, the dissolution of ye’elimite and the initial precipitation of hydrates (AFm/AFt and AH3) [15,16,17,18,19]. It can be seen in Fig. 3a that the cements containing ternesite have higher heat flow than the reference cement but the maxima of cement with 10% and 15% ternesite shifts to later times. After a period of dormancy, the first hydration peak of pure cement occurs at about 5 h, while the cement with 5% ternesite shows much weaker heat peak at 12 h. This peak is associated with the hydration reaction of ye’elimite with calcium sulfate to form ettringite and AH3 [20, 21]. However, no hydration peak is visible in the differential calorimetric curve for cements containing 10% and 15% ternesite. The second heat peak is only detected in the reference cement at about 12 h, and the heat release is mainly due to the precipitation of monosulfate occurring as a result of further rapid dissolution of ye’elimite and sulfate depletion [22]. The results indicate that the incorporation of ternesite inhibits the early hydration of CSA cement. As can be seen from the total heat output of CSA/ternesite mixtures shown in Fig. 3b, the cumulative heat flow values increases up to 6 h with the increase in the amount of ternesite. After this period of time, cement containing ternesite displays lower values of cumulative heat flow than the reference cement.
Setting time
The setting times of the cement paste are defined as the initiation of the solidification and subsequent hardening, which are mainly related to the rapid hydration reaction of calcium sulfate and calcium sulfoaluminate as well as the precipitation of hydrates (AFt, and/or AFm, AH3) [23, 24]. The initial and final setting times of CSA cements with the addition of ternesite are shown in Fig. 4. Generally, the initial setting of CSA cements occurs at the beginning of the accelerating heat pattern period, and the final setting times occurs before the corresponding heat output maxima [1]. The incorporation of ternesite has a considerable effect on setting times. Compared with the blank sample, the presence of ternesite causes a significant reduction in the setting times. It is apparent that the setting times become slightly longer with the increase in ternesite content. These phenomena are consistent with the previously discussed results on the calorimetric curves and the cumulative heats of CSA/ternesite combinations (Fig. 3). It can be seen that the initial heat flows in CSA/ternesite combinations are higher than that of pure CSA cement. Consequently, the setting times of CSA cement are shortened.
Compressive strength
Figure 4 displays the effect of ternesite on the compressive strengths development of CSA cement over 90 d. As illustrated in Fig. 5, the addition of ternesite decreases the compressive strength at early ages, especially when ternesite content increases to 15%, which should be attributed to the decreased CSA cement content. The early strengths of CSA cements develop quickly mainly due to ettringite formed during the hydration of calcium sulfoaluminate [25, 26]. After 28 days of hardening, the compressive strengths of the cement with 5% ternesite exhibit a significant increase and exceed that of the pure CSA cement. Although the compressive strength of the cement with 10% ternesite is slightly lower than that of the reference mortar over 56 d, a higher strength is attained at 90 d. However, 15% of ternesite remarkably decreases the compressive strength of CSA cements. The result indicates that 5% of ternesite is most favorable for the later strength development of CSA cements. This may be related to the hydration of ternesite. Ternesite is often considered to decrease the setting rate of CSA cement, potentially acting as an inert phase with very low reactivity degree [9, 27,28,29]. However, ternesite can be activated by the aluminum released during ye’elimite hydration and is more reactive than belite [11, 30, 31]. The formation of cementitious phases from the hydration of ternesite at later ages contributes to the mechanical strength of CSA cements. Therefore, ternesite can be used as a reactive additive in CSA cements.
Dimensional stability
Since ettringite formation in Portland cement is usually linked to expansion and degradation in forms of sulfate attack including delayed ettringite formation and external attack [32], the dimensional stability of CSA cement is also related to significant ettringite formation after hardening and needs to be paid attention. The ettringite formation from the hydration of ternesite may be associated with expansion of the set mass. Dimensional changes of CSA cement mortars at varying contents of ternesite are shown in Fig. 6. When cured in water, the CSA cements with the addition of ternesite show limited expansion and the linear expansion ratio is within 4 × 10−4. Moreover, the slight difference can be observed at early ages. Compared with the reference cement mortar, the cement mortar with 15% ternesite exhibits a higher expansion rate, while the specimens with 5% and 10% ternesite replacement to the cement result in similarly lower expansions after 7 days of hardening. While cured in air, the addition of ternesite contributes to lower drying shrinkage. The lowest shrinkage actually appears for the mortar with 10% ternesite, while the mortars with 5% and 15% ternesite experience similarly moderate shrinkage. Though ternesite present in the hydrating CSA cement may be the source of “delayed ettringite formation,” the results in this investigation indicate that ternesite with appropriate content is harmless to the volume stability of CSA cement.
Hydration products
Ternesite has been also identified as a secondary phase in CSA clinkers and traditionally been considered as an inert phase [33, 34]. The previous study of Shen et al. showed that the hydration of pure ternesite commenced at 90 days and gypsum was formed as a new crystalline phase [11]. Recent studies have shown that the aluminum released during ye’elimite hydration has a strong influence on ternesite reactivity, as the dissolution rate of ternesite is affected by the availability of Al(OH)−4 within the pore solution [8]. In order to investigate the effect of ye’elimite on the hydraulic behavior of ternesite, 30% of ye’elimite was added to the synthesized ternesite. Ye’elimite was synthesized by firing the stoichiometric mixture of laboratory-grade CaCO3, Al2O3 and CaSO4 at 1350 °C for 2 h. The obtained sample was ground to pass through a 80-μm sieve. The phase purity determined by applying Rietveld refinement to the XRD pattern was 100% calcium sulfoaluminate. The XRD patterns of the hydrated pastes up to 90 days are demonstrated in Fig. 7. The hydration of ternesite is characterized by the formation of ettringite at 28 days, and its amount is progressively increasing by further ternesite depletion. With hydration proceeding, the characteristic peak of ye’elimite decreases obviously. The characteristic peak of gypsum can be found at 56 days but disappears after 90 days of hydration. This indicates that the hydration of ternesite in the presence of ye’elimite is accompanied with the formation of gypsum followed by Al(OH)−4 consumption, and the precipitation of ettringite is promoted. Additionally, monosulfate is detected at 90 days. It can be concluded that the hydration reactivity of ternesite is activated by ye’elimite, which is consistent with the finding of previous work.
SEM images of the pastes at 90 days of hydration were also investigated. As shown in Fig. 8, it is clearly revealed that ettringite-like needles can be detected in 70% ternesite/30% ye’elimite hydrated system. This confirms that the addition of ye’elimite stimulates the hydration of ternesite and favors the formation of ettringite, which is consistent with the XRD results.
In order to assess the hydration process, the mineral compositions of hardened cement pastes were characterized by XRD and the patterns are shown in Fig. 9. Anhydrite has been almost fully consumed, and there are still some ye’elimite remains in the paste. Ettringite has formed as a new crystalline phase in all tested samples. No characteristic peaks of portlandite can be found in the hydration products. Traces of aluminum hydroxide (AH3) are not detectable due to its poor crystallized structure [35]. As can be seen from Fig. 9a, with the increase in ternesite content, the diffraction peaks of ye’elimite and ettringite decrease obviously. This can be ascribed to the dilution produced by the addition of ternesite. After 28 days, the XRD patterns exhibit some differences from that of 7 days. The presence of 5% ternesite promotes the formation of ettringite, while the intensity of ettringite diffraction peaks decreases obviously for the cements with 10% and 15% ternesite. This is consistent with the development of strength. Figure 9c reveals a prominent increase in ettringite formation after 90 days of hydration. There is no obvious depletion of belite from 7 d to 90 d. It is interesting to note that gypsum from the hydration of ternesite is detected when 10% of ternesite is added. Additionally, the XRD patterns exhibit no prominent difference from that of 28 days. This indicates that the appropriate addition of ternesite can favor the formation of ettringite at later ages and the cement dilution can be compensated by the hydration of ternesite.
SEM images of the cement pastes with ternesite for 7 days and 28 days are shown in Fig. 10. Column-shaped ettringite crystals can be observed in the cement pastes due to the hydration reaction of ye’elimite with anhydrite. From the XRD analysis of hydration products, it is observed that the hydration of ternesite can promote the formation of ettringite and thus the later strength development. It can be found that there are no obvious differences in the ettringite crystal micromorphology between the cement containing 5% ternesite and the blank sample. The results show that the addition of ternesite does not affect the crystallization of ettringite, which is not in accordance with the previous work [9]. This may be due to the difference between the addition of ternesite to CSA cements and the formation of ternesite in CSA clinkers.
Conclusions
This study presents the feasibility of using ternesite as an additive of calcium sulfoaluminate (CSA) cements, targeting at improving cement performance. The study aims to characterize the activation of calcium sulfoaluminate on the reactivity of ternesite, following the setting time, mechanical strength, dimensional stability and hydration kinetics of cements containing ternesite. The following conclusions can be drawn from this investigation:
- 1.
The incorporation of ternesite shortens the initial and final setting times of CSA cements, but increasing the substitution amount of ternesite is able to slightly prolong the setting times.
- 2.
The addition of ternesite decreases the compressive strength at early ages. After 28 days of hardening, 5% of ternesite contributes higher compressive strength compared to plain CSA cement while the cements with 10% and 15% ternesite suffer a strength decrease. A slightly higher strength is attained for the cement with 10% ternesite at 90 days.
- 3.
Compared with the reference cement mortar, the cement mortar with 15% ternesite exhibits a higher expansion rate, while the presence of ternesite results in lower drying shrinkage. The use of ternesite as an additive in CSA cements is harmless to the volume stability of CSA cements.
- 4.
The hydration activity of ternesite can be effectively activated by ye’elimite, and the hydration of ternesite in the presence of ye’elimite is accompanied with the formation of gypsum followed by the precipitation of ettringite. The addition of ternesite has affected the hydration products of CSA cements and has promoted the formation of ettringite at later ages.
References
Ioannou S, Reig L, Paine K, Quilin K. Properties of a ternary calcium sulfoaluminate-calcium sulfate-fly ash cement. Cem Concr Res. 2014;56(2):75–83.
Bernardo G, Telesca A, Valenti GL. A porpsimetric study of calcium sulfoaluminate cement pastes cured at early ages. Cem Concr Res. 2006;36(6):1042–7.
Péra J, Ambroise J. New applications of calcium sulphoaluminate cement. Cem Concr Res. 2004;34(4):671–6.
Beretka J, Vito BD, Santoro L, Sherman N, Valenti GL. Utilisation of industrial wastes and by-products for the synthesis of special cements. Resour Conserv Recycl. 1993;9(3):179–90.
Beretka J, Vito BD, Santoro L, Sherman N, Valenti GL. Hydraulic behavior of calcium sulfoaluminate-based cements derived from industrial process wastes. Cem Concr Res. 1993;23(5):1205–14.
Hou PK, Qian JS, Wang Z, Deng C. Production of quasi-sulfoaluminate cementitious materials with electrolytic manganese residue. Cem Concr Compos. 2012;34(2):248–54.
Wang YM, Su MZ, Zhan L. Sulfoaluminate cement. 1st ed. Beijing: Beijing University of Technology Press; 1999 [in Chinese].
Bullerjahn F, Schmitt D, Ben Haha M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem Concr Res. 2014;59(5):87–95.
Sherman N, Beretka J, Santoro L, Valenti GL. Long-term behavior of hydraulic binders based on calcium sulfoaluminate and calcium sulfosilicate. Cem Concr Res. 1995;25(1):113–26.
Skalamprinos S, Galan I, Hanein T, Glasser F. Enthalpy of formation of ye’elimite and ternesite. J Therm Anal Calorim. 2018;131(3):2345–59.
Shen Y, Qian JS, Huang YB, Yang DY. Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cem Concr Compos. 2015;63:67–75.
Ben Haha M, Bullerjahn F, Zajac M. On the reactivity of ternesite. In: 14th International congress on the chemistry of cement, Beijing, China; 2015.
Kacimi L, Cyr M, Clastres P. Synthesis of α’L-C2S cement from fly-ash using the hydrothermal method at low temperature and atmospheric pressure. J Hazard Mater. 2010;181(1):593–601.
Costa EBD, Rodríguez ED, Bernal SA, Provis JL, Gobbo LA, Kirchheim AP. Production and hydration of calcium sulfoaluminate-belite cements derived from aluminium anodising sludge. Constr Build Mater. 2016;122:373–83.
Winnefeld F, Barlag S. Influence of calcium sulfate and calcium hydroxide on the hydration of calcium sulfoaluminate clinker. Zkg Int. 2009;62(12):42–53.
Zhang L, Glasser FP. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv Cem Res. 2002;14(4):141–55.
Hargis CW, Kirchheim AP, Monteiro PJM, Gartner EM. Early age hydration of calcium sulfoaluminate (synthetic ye’elimite, \({\text{C4A3}}\overline{\text{S}}\)) in the presence of gypsum and varying amounts of calcium hydroxide. Cem Concr Res. 2013; 48:105-15.
Álvarez-Pinazo G, Cuesta A, García-Mate M, Santacruz I, Losilla ER, De la Torre AG, León-Reina L, Aranda MAG. Rietveld quantitative phase analysis of ye’elimite-containing cements. Cem Concr Res. 2012;42(7):960–71.
Zhang Y, Zhang ZH, Li WF, Wang H, Shen XD. Welan gum retards the hydration of calcium sulfoaluminate. J Therm Anal Calorim. 2017;130(2):899–908.
Bullard JW, Jennings HM, Livingston RA, Nonat A, Scherer GW, Schweitzer JS, Scrivener KL, Thomas JJ. Mechanisms of cement hydration. Cem Concr Res. 2011;41(12):1208–23.
Dovál M, Paloui M, Kovár V. Heat evolution and mechanisms of hydration in CaO-Al2O3-SO3 system. Ceram Silik. 2005;49(2):104–8.
Cuberos AJM, De la Torre AG, Alvarez-Pinazo G, Martín-Sedeño MC, Schollbach K, Pöllmann H, Aranda MAG. Active iron-rich belite sulfoaluminate cements: clinkering and hydration. Environ Sci Technol. 2010;44(17):6855–62.
Ma B, Ma M, Shen XD, Li XR, Wu XD. Compatibility between a polycarboxylate superplasticizer and the belite-rich sulfoaluminate cement: setting time and the hydration properties. Constr Build Mater. 2014;51(2):47–54.
Winnefeld F, Lothenbach B. Hydration of calcium sulfoaluminate cements-Experimental findings and thermodynamic modelling. Cem Concr Res. 2010;40(8):1239–47.
Chen IA, Juenger MCG. Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cem Concr Compos. 2012;34(8):893–902.
Huang YB, Qian JS, Liang J, Liu N, Li FL, Shen Y. Characterization and calorimetric study of early-age hydration behaviors of synthetic ye’elimite doped with the impurities in phosphogypsum. J Therm Anal Calorim. 2016;123(2):1545–53.
Belz G, Beretka J, Marroccoli M. Use of fly ash, blast furnace slag and chemical gypsum for the synthesis of calcium sulphoaluminate-based cements. Spec Publ ACI. 1995;153:513–30.
Makhmudova V, Iskandarova M, Ivanova Y, Chernev G, Ruziev N. Synthesis and properties of sulphoferrite calcium clinkers and low temperature cements on their basis. J Univ Chem Technol Metall. 2011;46(2):151–4.
Tadzhiev TK, Atakuziev TA, Tadzhiev FK. Hardening of anhydrous calcium sulfoaluminate and sulfosilicate. UDC. 1973;691:1434–7.
Dienemann W, Schmitt D, Bullerjahn F, Ben Haha M. Belite-calcium sulfoaluminate-ternesite (BTC) - a new low carbon clinker technology. Cem Int. 2013;11(4):100–9.
Montes M, Pato E, Carmona-Quiroga PM, Blanco-Varela MT. Can calcium aluminates activate ternesite hydration. Cem Concr Res. 2018;103:204–15.
Taylor HFW. Cement Chemistry. 2nd ed. London: Thomas Telford; 1997.
Beretka J, Cioffi R, Marroccoli M, Valenti GL. Energy-saving cements obtained from chemical gypsum and other industrial wastes. Waste Manag. 1996;16(1–3):231–5.
Beretka J, Marroccoli M, Sherman N, Valenti GL. The influence of \({\text{C4A3}}\overline{\text{S}}\) content and WS ratio on the performance of calcium sulfoaluminate-based cements. Cem Concr Res. 1996; 26(26): 1673-81.
Winnefeld F, Barlag S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J Therm Anal Calorim. 2010;101(3):949–57.
Acknowledgements
The authors give thanks to the financial support from China Postdoctoral Science Foundation (2017M611924) and Natural Science Foundation of Jiangsu Province (BK20170505). A Project Funded by College Natural Science Research of Jiangsu Province (17KJB560013) should also be appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, Y., Chen, X., Zhang, W. et al. Effect of ternesite on the hydration and properties of calcium sulfoaluminate cement. J Therm Anal Calorim 136, 687–695 (2019). https://doi.org/10.1007/s10973-018-7685-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7685-x