Abstract
This work explores the effect of lithium salt on the properties of calcium sulfoaluminate (CSA) cements where different proportions of gypsum are added. Experiments comprising hydration heat release, setting time, mechanical strength and hydration products are studied. The results showed that the addition of Li2CO3 significantly accelerates the early hydration and decreases the setting time of CSA cement whatever the gypsum content added to cement. When the content of Li2CO3 increases, the setting time decreases accordingly. When gypsum is incorporated, the compressive strength development of the mortars with Li2CO3 suffers a decrease, especially after one day of hydration. When the amount of gypsum increases, the later strength of the mortars with Li2CO3 exhibits no obvious decrease. The addition of Li2CO3 inhibits \({\text{C4A3}}\overline{{\text{S}}}\) hydration of CSA cement comprising 10% gypsum at 1 day but promotes ettringite formation after 28 days of hydration. In contrast, the presence of Li2CO3 promotes \({\text{C4A3}}\overline{{\text{S}}}\) hydration of CSA cement without gypsum addition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calcium sulfoaluminate (CSA) cement is receiving great attention due to their environmental benefits in contrast with Portland cement (PC) [1, 2]. CSA cement consists ye’elimite (\({\text{C4A3}}\overline{{\text{S}}}\)) and different proportions of belite (C2S), calcium sulfate (\({\text{C}}\overline{{\text{S}}}\)) and aluminoferrite (C4AF). CSA cement has excellent properties, such as high early strength, rapid setting, low permeability and shrinkage compensating, which are highly dependent on the hydration of \({\text{C4A3}}\overline{{\text{S}}}\) with calcium sulfate [3,4,5]. For those reasons, CSA cement represents a sustainable alternative to PC. Currently, CSA cement is mainly applied in winter construction projects, precast products and emergency repairs [6]. Although CSA cement is receiving increasing attention, many barriers prevent their industrial-scale production and widespread usage [7].
The hydration mechanism of CSA cement is well characterized. Due to the reaction of \({\text{C4A3}}\overline{{\text{S}}}\) with calcium sulfate, ettringite and aluminum hydroxide form as the primary early age hydration products. As soon as calcium sulfate is deficient, monosulfate forms together with aluminum hydroxide [8,9,10]. The quantities of precipitated ettringite and monosulfate are thus closely related to the type and amount of added calcium sulfate. Moreover, the content of added calcium sulfate significantly affects the distribution of hydration products and the properties of CSA cement-based materials [9]. It is found that calcium sulfate speeds up the hydration kinetics of \({\text{C4A3}}\overline{{\text{S}}}\) at early ages [11]. Additionally, the strength development and dimensional stability of CSA cement are also sensitive to the content of calcium sulfate. If properly formulated, a great deal of CSA cement-based materials can be manufactured [12].
CSA cement is mostly used in small-scale construction engineering due to lack of formalized design guidance. When CSA cement is applied in quick repairs, accelerators are necessary to meet the requirements of rapid setting and hardening in quick repairs. It is known that lithium salts can speed up the hydration of calcium aluminate cement as well as CSA cement [13,14,15]. The acceleration may be related to the fast emergence of Li-containing aluminum hydroxide. Its occurrence provides seeds for promoting the formation of amorphous aluminum hydroxide, which conversely accelerates the hydration process of cements [16]. Some work has been done to discuss the effect of lithium ions on the hydration of CSA cement at early ages [16]. The results showed that the hydration is sped up by a rise in the lithium concentration up to 30 μmol Li/g of the CSA cement and then levels off. Therefore, a small amount of lithium is enough for the acceleration of CSA cement. The acceleration of cement hydration can cause the changes of the macro-properties, like setting, hardening and the mechanical properties. However, the deep study on the development of the properties of CSA cement with lithium salts is very limited. Thus, it is important to incorporate the appropriate amount of lithium salts to improve the performance of CSA cement.
As calcium sulfates and lithium salts affect the early hydration of CSA cement by different mechanisms, the presence of sulfate ions may change the hydration process accelerated by lithium ions. Consequently, the objective of this article is to discuss the effect of lithium salt on the hydration and properties of CSA cement with variable gypsum contents. The early hydration of cement pastes was characterized by the heat evolution and setting time. Moreover, the mechanical strength, hydrates assemblage and microstructure were also investigated. This study will provide new insight for the application of CSA cements in building technologies.
Experimental
Materials
A CSA cement clinker and natural gypsum (NG) were utilized in this work to prepare the cements. The CSA cement clinker was manufactured by Liujiu Cement Co., Ltd (China). The chemical compositions and phase contents of CSA cement clinker and NG are listed in Table 1. The main compounds of the CSA cement clinker are \({\text{C4A3}}\overline{{\text{S}}}\) and C2S. In addition, the CSA cement clinker contains other minor compounds including C4AF, mayenite (C12A7) and \({\text{C}}\overline{{\text{S}}}\). It has a Blaine fineness of 3500 cm2 g−1. Analytical grade lithium carbonate (Li2CO3) was used as the accelerator.
Mix design and methods
Four binder compositions were investigated. The CSA cement clinker was replaced by an increased amount of NG from 5%, 10% and 15 mass%, and marked with the symbol of C1, C2 and C3, respectively. The CSA cement clinker was considered as a reference (marked as C0). The amount of Li2CO3 was by mass of clinker-gypsum combinations, e.g., 0.00%, 0.03%, 0.07% and 0.1%. The lithium salt was dissolved in advance into the mixing water to ensure a uniform dispersion of the accelerator in the sample.
The setting time was measured by a standard Vicat apparatus according to Chinese standard GB/T 1346-2001. The standard mortars consisted of the cement, sand and water with the proportion of 1:3:0.5. The mortars were then cast in the molds (40 mm × 40 mm × 160 mm). The molds were transferred to the curing room where the temperature and relative humidity (RH) were 20 °C and 95%, respectively. After one day of curing, the mortars were demolded and continuously cured in water at 20 °C. At the specific ages, the mortars were utilized for mechanical strength test on the basis of Chinese standard GB/T 17671-1999. The value of the compressive strength was the average of six samples. With regard to the volume stability measurements, the mortars were also composed of the cement, sand and water with the proportion of 1:3:0.5. The mortars were cast in the molds ( 25 mm × 25 mm × 280 mm). Then, the molds were cured by using the same procedure described above. After one day of curing, the mortars were demolded, and the initial length was measured immediately. The mortars were subsequently transferred to cure in air and water, respectively, and the length changes were measured at the specific testing ages.
The hydration heat release was examined by isothermal conductive calorimetry. Experiments were conducted at 20 °C by employing an eight channel TAM AIR conductive calorimeter. The water-cement ratio was 0.5. The measurements were conducted at the atmosphere of air.
The cement pastes were prepared at a constant water-cement ratio of 0.4. At the specific period, the cement pastes were crushed and immersed in ethanol for 48 h in order to stop the cement hydration. Then, the samples were transferred to a vacuum desiccator with a temperature of 40 °C. Finally, the samples were ground to pass through a 200 mesh sieve for X-ray diffraction (XRD) analysis.
The XRD patterns were recorded on an X-ray diffractometer (Bruker D8 Advance diffractometer; Cu Kα radiation). The X-ray tube was conducted at 40 kV and 40 mA. Step scans were carried out in the range of 5–65o with a step size of 0.02°. TOPAS 4.2 software was applied for the Rietveld quantitative analysis of the samples. Microstructure and morphology were identified by scanning electron microscopy (SEM).
Results
Hydration heat release
Figure 1 depicts the hydration heat release of CSA cements with the addition of Li2CO3. The initial peak which occurs after the cement gets in touch with the water is ascribed to the wetting and dissolution of the samples as well as the occurrence of the hydration reactions [17,18,19]. The first hydration heat release is possibly ascribed to the dissolution of \({\text{C4A3}}\overline{{\text{S}}}\) and the initial formation of hydrates [20,21,22]. Sample C0 shows a short dormant period after the initial peak until about 1 h. Afterward, sample C0 is characterized by two peaks which occur at about 1.9 h and 4 h, respectively. The first hydration peak is corresponding to the consumption of gypsum and the formation of ettringite together with aluminum hydroxide [23, 24]. The second peak presents the period of a continuous hydration reaction after the gypsum depletion and monosulfate and more ettringite form as the main hydration products [25]. When Li2CO3 is added to sample C0, the dormant period disappears and the heat flow reaches the first maximum around 15 min of hydration, indicating a stronger acceleration of hydration. Moreover, this heat peak is more intense than the corresponding one at about 1.9 h in the case of sample without Li2CO3 addition. The subsequent hydration peak occurs at about 2.5 h, which is earlier than that of sample without Li2CO3 (4 h). This reflects the trend of Li2CO3 to accelerate the formation of sulfoaluminate hydrates. Besides, the third heat peak of sample C0 with Li2CO3 occurs after 5 h of hydration. When 10% gypsum is added to CSA clinkers, the initial peak is more intense and the dormant period is significantly shortened. After the dormant period, sample C2 is characterized by two peaks with their maxima at 0.7 h and 1.6 h with a shoulder at 2.1 h, respectively. It can be found that the presence of gypsum remarkably accelerates the hydration of CSA cements. In comparison, the hydration peaks of sample C2 with Li2CO3 occur at 0.4 h and 1.5 h with a shoulder at 2.6 h, respectively, which are also earlier than these of sample without Li2CO3. It can be seen that the addition of Li2CO3 increases the heat flow of the first maximum but decreases the heat flow of the subsequent peaks for sample C2, while for sample C0, the presence of Li2CO3 enhances the heat flow of all hydration peaks.
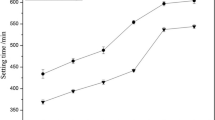
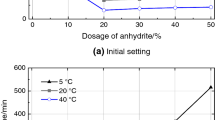
Setting time
The setting time of CSA cements with different concentrations of Li2CO3 is demonstrated in Fig. 2. The addition of Li2CO3 significantly affects the setting time of CSA cements. The incorporation of Li2CO3 results in a prominent decrease in the setting time. This indicates that Li2CO3 accelerates the setting process of CSA cements. The phenomena agree with the findings of the heat release of CSA cements (Fig. 1). The setting time of CSA cement was observed to be corresponding to the time of the peak of hydration heat [26]. It is observed that the first heat peak for samples with Li2CO3 is higher and occurs faster than that of samples without Li2CO3. Therefore, the earlier precipitation of the hydrates causes the setting time of CSA cements with Li2CO3 to become shorter. Moreover, whatever the gypsum content added to the cements (0%, 5%, 10% or 15%), the setting time decreases with the increased amount of Li2CO3. When the dosage of Li2CO3 increases to 0.07%, there is a slight decrease in the setting time. The initial setting time and final setting time of sample C0 are shortened to 8 min and 13 min in the presence of 0.1% Li2CO3, respectively. The presence of gypsum causes a decrease on the setting time of CSA cements in the absence of Li2CO3. When the content increases from 0 to 10%, the initial setting time of CSA cements decreases from 37 to 23 min, while the same content of gypsum significantly decreases the final setting time from 64 to 38 min. However, the setting time of CSA cements is slightly prolonged when the cement contains 15% gypsum.
Mechanical strength
The effects of Li2CO3 on the compressive strength development of CSA cements are demonstrated in Fig. 3. The main strength increase is observed after 7 days of hydration. The CSA cements show rapid early strength because the hydration reactions of \({\text{C4A3}}\overline{{\text{S}}}\) and gypsum initiate rapidly and thus lead to the precipitation of ettringite and aluminum hydroxide [27]. The effect of Li2CO3 on the compressive strength development varies for different cements. After one day of hydration, the strength development of sample C0 with the addition of Li2CO3 is almost equal to that of the reference cement. When the hydration proceeds from 3 to 28 days, adding Li2CO3 into the CSA cements slightly decreases the strength of the mortars. The lowest strength is recorded for specimen with 0.07% Li2CO3. The incorporation of gypsum enhances the compressive strength of the mortars, particularly at 1 day. It can be seen that a dosage of 5% gypsum is optimum for the compressive strength of CSA cements. In the presence of gypsum, the strength of the mortars with the addition of Li2CO3 suffers a decrease, especially after 1 d of hydration. With increasing Li2CO3 contents, it can be found that there are no obvious differences in the strength development except for sample C1 with 0.07% Li2CO3 at 28 d and sample C2 with 0.03% Li2CO3 at 7 d. The compressive strength of sample C2 with the presence of Li2CO3 becomes slightly higher than the reference cement at 28 d. As gypsum content increases, the later strength of the mortars with Li2CO3 exhibits no obvious decrease.
Volume stability
CSA cements have shown shrinkage compensation during the manufacturing process [28]. The hydration of \({\text{C4A3}}\overline{{\text{S}}}\) with calcium sulfate forms ettringite and aluminum hydroxide, which results in the expansion and early age strength development of CSA cements [29]. Moreover, if significant ettringite forms after hardening, cracking may occur [30,31,32]. Thus, it is worth to study the volume stability of CSA cements. Figures 4 and 5 display the dimensional stability of mortars for C0 and C2 system with different Li2CO3 contents. As can be seen from Fig. 4a, when cured in air, the Li2CO3-added samples (C0) exhibit relatively lower drying shrinkage compared with the blank sample. All mortars exhibit rapid shrinkage up to 7 days, and then, the shrinkage achieves a plateau until 28 days. The mortar with 0.03% Li2CO3 yields the lowest shrinkage among the investigated mortars. As expected, much lower drying shrinkage is obtained for the mortars due to the incorporation of gypsum. When gypsum is incorporated into the cement, the effect of Li2CO3 on the drying shrinkage is not significant. The mortar with 0.1% Li2CO3 always obtains the lowest drying shrinkage within the curing age. After 7 d of hydration, all the mortars exhibit similar drying shrinkage.
When cured in water, 0.03% and 0.1% of Li2CO3 result in much higher expansion rate of sample C0, while the expansion rate of sample with 0.07% Li2CO3 is similar to that of the reference mortar. The addition of gypsum results in much higher expansion of the mortars. Enhancing the content of gypsum leads to more ettringite formation, which increases the risk of expansion [32]. However, the expansion rates of sample C2 are within 3 × 10−4 at 28 d which does not affect the volume stability of CSA cements. When gypsum is incorporated into the cement, 0.07% and 0.1% of Li2CO3 result in much higher expansion rate of sample C2, while the expansion rate of sample with 0.03% Li2CO3 is similar to the reference cement mortar. The results in this investigation indicate that the addition of Li2CO3 does not have adverse effect on the volume stability of CSA cement.
Hydration products
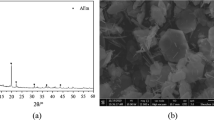
The XRD patterns of cements containing different gypsum contents after 1 d are presented in Fig. 6. It is observed from the XRD patterns that \({\text{C4A3}}\overline{{\text{S}}}\) and C2S do not react completely in the pastes. All samples display the formation of ettringite even in the paste without gypsum addition. The trace of aluminum hydroxide is not found by XRD due to its poor crystallized structure [11, 33]. Due to the rise of gypsum content, the intensity of ettringite peaks increases significantly, while the peaks of \({\text{C4A3}}\overline{{\text{S}}}\) decrease. In the cement paste free from gypsum, a small amount of ettringite has formed after 1 d. However, precipitation of calcium monosulfoaluminate hydrate is not detected. Gypsum can be clearly identified in the paste with 15% gypsum, showing that the added gypsum is excessive at early ages.
Figure 7 shows the XRD patterns of the C2 system with different Li2CO3 contents after 1 d and 28 d. It can be found that the dominant hydration product of the C2 system is still ettringite in the presence of Li2CO3. The Li2CO3 dosage affects the hydration of \({\text{C4A3}}\overline{{\text{S}}}\). After 1 day of hydration, the characteristic peaks of \({\text{C4A3}}\overline{{\text{S}}}\) appear to become strong with the addition of Li2CO3. Moreover, gypsum can be clearly observed in the paste containing 0.03% and 0.07% Li2CO3. The finding shows that the addition of Li2CO3 inhibits the hydration of \({\text{C4A3}}\overline{{\text{S}}}\) at 1 day. After 28 days of hydration, the XRD patterns show some distinctions. An increase in ettringite can be clearly observed and the addition of Li2CO3 promotes the ettringite formation. Traces of gypsum are not noticed.
Figure 8 shows the XRD patterns of hydrated C0 and C3 with the addition of Li2CO3 at 1 d. It can be found that the intensity of ettringite peaks significantly increases with the presence of Li2CO3, while the intensity of \({\text{C4A3}}\overline{{\text{S}}}\) peaks decreases correspondingly for sample C0. In contrast, the intensity of ettringite peaks slightly decreases with the addition of Li2CO3 for sample C3. Moreover, an increase in gypsum can be observed in the sample containing Li2CO3. The results shown in Figs. 7 and 8 are identical to the compressive strength development shown in Fig. 3.
Microstructures of the cement pastes with Li2CO3 are presented in Figs. 9 and 10. Based on the results of hydration products, it is discovered that after one day of hydration, a small amount of ettringite has formed in the cement paste free from gypsum. The formed ettringite may be embedded in the hardened paste as given in Fig. 9. As shown in Fig. 10, needle-shaped ettringite crystals can be discovered in the cement paste with 10% gypsum. It can be found that the presence of Li2CO3 does not change the morphology of ettringite crystals when compared to the reference sample.
Conclusions
The influence of lithium salt on the performance of CSA cements was studied. The following conclusions are summarized based on this study:
-
(1)
The addition of Li2CO3 remarkably accelerates the hydration process of CSA cement at early ages and decreases the setting time of CSA cement. Whatever the gypsum content added to the cement, the setting time of CSA cement decreases with the increased amount of Li2CO3.
-
(2)
In the presence of gypsum, the compressive strength development of the mortars with the incorporation of Li2CO3 suffers a decrease, especially after one day of hydration. As gypsum content increases, the later strength of the mortars with Li2CO3 exhibits no obvious decrease.
-
(3)
Compared with the reference mortar, the addition of Li2CO3 results in lower drying shrinkage and higher expansion rate of sample C0. In the presence of Li2CO3, the addition of gypsum causes much lower drying shrinkage and higher expansion rate of the mortars.
-
(4)
The addition of Li2CO3 does not alter the hydration products, but inhibits the hydration of \({\text{C4A3}}\overline{{\text{S}}}\) of CSA cement containing 10% gypsum at 1 day. In contrast, the presence of Li2CO3 promotes the precipitation of ettringite after one day of hydration.
References
Schneider M, Romer M, Tschudin M, Bolio H. Sustainable cement production-present and future. Cem Concr Res. 2011;41(7):642–50.
Shi CJ, Fernández Jiménez A, Palomo A. New cements for the 21st century: the pursuit of an alternative to Portland cement. Cem Concr Res. 2011;41(7):750–63.
Quillin K. Performance of belite-sulfoaluminate cements. Cem Concr Res. 2001;31(9):1341–9.
Lu XL, Ye ZM, Wang SX, Du P, Li CH, Cheng X. Study on the preparation and properties of belite-ye’elimite-alite cement. Constr Build Mater. 2018;182:399–405.
Bescher E, Rice EK, Ramseyer C, Roswurm S. Sulfate resistance of calcium sulphoaluminate cement. J Struct Integr Main. 2016;1(3):131–9.
Shi C, Zou XW, Wang P. Influences of ethylene-vinyl acetate and methylcellulose on the properties of calcium sulfoaluminate cement. Constr Build Mater. 2018;193:474–80.
Lisa EB, Prasanth A, Robert DM, Ley MT, Berke N, Kurtis KE. Alternative cementitious materials: challenges and opportunities. ACI Spec Publ. 2015;305(15):1–10.
Winnefeld F, Lothenbach B. Hydration of calcium sulphoaluminate cements-experimental findings and thermodynamic modelling. Cem Concr Res. 2010;40(8):1239–47.
Pelletier L, Winnefeld F, Lothenbach B. The ternary system Portland cement-calcium sulphoaluminate clinker-anhydrite: hydration mechanism and mortar properties. Cem Concr Compos. 2010;32(7):497–507.
Pelletier-Chaignat L, Winnefeld F, Lothenbach B, Le Saout G, Jörg Müller C, Famy C. Influence of the calcium sulphate source on the hydration mechanism of Portland cement-calcium sulphoaluminate clinker-calcium sulphate binders. Cem Concr Compos. 2011;33(5):551–61.
Winnefeld F, Barlag S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J Therm Anal Calorim. 2010;101(3):949–57.
Glasser FP, Zhang L. High-performance cement matrices based on calcium sulphoaluminate-belite compositions. Cem Concr Res. 2001;31(12):1881–6.
Capmas A, Damidot D, Rettel A. Action of admixtures on Fondu cement: part I Lithium and sodium salts compared. Adv Cem Res. 1996;8(31):111–9.
Damidot D, Sorrentino D, Rettel A, Capmas A. Action of admixtures on Fondu cement: part II effect of lithium salts on the anomalous setting time observed for temperatures ranging from 18 to 35 °C. Adv Cem Res. 1997;9(35):127–34.
Millard MJ, Kurtis KE. Effect of lithium nitrate admixture on early-age cement hydration. Cem Concr Res. 2008;38(4):500–10.
Céline CDC, Dhoury M, Champenois JB, Mercier C, Damidot D. Physico-chemical mechanisms involved in the acceleration of the hydration of calcium sulfoaluminate cement by lithium ions. Cem Concr Res. 2017;96:42–51.
Bullerjahn F, Schmitt D, Ben HM. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem Concr Res. 2014;59:87–95.
Xu JT, Chen JW, Lu DY, Xu ZZ, Hooton RD. Effect of dolomite powder on the hydration and properties of calcium sulfoaluminate cements with different gypsum contents. Constr Build Mater. 2019;225:302–10.
Zajac M, Skocek J, Bullerjahn F, Ben HM. Effect of retarders on the early hydration of calcium-sulpho-aluminate (CSA) type cements. Cem Concr Res. 2016;84:62–75.
Bullerjahn F, Boehm-Courjault E, Zajac M, Ben Haha M, Scrivener K. Hydration reactions and stages of clinker composed mainly of stoichiometric ye’elimite. Cem Concr Res. 2019;116:120–33.
Zajac M, Skocek J, Stabler C, Bullerjahn F, Ben HM. Hydration and performance evolution of belite-ye’elimite-ferrite cement. Adv Cem Res. 2019;31(3):124–37.
Bullerjahn F, Zajac M, Ben Haha M, Scrivener KL. Factors influencing the hydration kinetics of ye’elimite; effect of mayenite. Cem Concr Res. 2019;116:113–9.
Chen IA, Juenger MCG. Synthesis and hydration of calcium sulfoaluminate-belite cements with varied phase compositions. J Mater Sci. 2011;46(8):2568–77.
Bullard JW, Jennings HM, Livingston RA, Nonat A, Scherer GW, Schweitzer JS, Scrivener KL, Thomas JJ. Mechanisms of cement hydration. Cem Concr Res. 2011;41(12):1208–23.
Jansen D, Spies A, Neubauer J, Ectors D, Goetz-Neunhoeffer F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem Concr Res. 2017;91:106–16.
Lisa EB, Kimberly EK. Influence of set retarding admixtures on calcium sulfoaluminate cement hydration and property development. Cem Concr Res. 2018;104:105–13.
Péra J, Ambroise J. New applications of calcium sulfoaluminate cement. Cem Concr Res. 2004;34(4):671–6.
Buzzi L, Canonico F, Telesca A, Valenti GL. High-performance and low-CO2 cements based on calcium sulphoaluminate. ZKG Int. 2010;63(5):39–45.
Kasselouri V, Tsakiridis P, Malami Ch, Georgali B, Alexandridou C. A study on the hydration products of a non-expansive sulfoaluminate cement. Cem Concr Res. 1995;25(8):1726–36.
Ogawa K, Roy DM. \({\text{C4A3}}\overline{{\text{S}}}\) hydration, ettringite formation, and its expansion mechanisms: III. Effect of CaO, NaOH, and NaCl; conclusions. Cem Concr Res. 1982;12(2):247–256.
Huang YB, Qian JS, Liu CZ, Liu N, Shen Y, Ma Y, Sun HQ, Fan YR. Influence of phosphorus impurities on the performances of calcium sulfoaluminate cement. Constr Build Mater. 2017;149:37–44.
Chen IA, Hargis CW, Juenger MCG. Understanding expansion in calcium sulfoaluminate-belite cements. Cem Concr Res. 2012;42(1):51–60.
Song F, Yu ZL, Yang FL, Lu YN, Liu YF. Microstructure of amorphous aluminum hydroxide in belite-calcium sulfoaluminate cement. Cem Concr Res. 2015;71:1–6.
Acknowledgements
The work is supported by National Nature Science Foundation of China (No. 51802279) and Natural Science Foundation of Jiangsu Province (BK20170505). Financial support from Qing Lan Project of Yangzhou University is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, Y., Zhang, W., Wang, P. et al. Influence of lithium salt on the performance of calcium sulfoaluminate cement. J Therm Anal Calorim 147, 3043–3051 (2022). https://doi.org/10.1007/s10973-021-10715-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10715-4