Abstract
The ignition reaction of Mg/KNO3 was improved with addition of Ag/CNTs nanocomposite as catalyst. The nanoparticles of Ag(0) was deposited on the multi-walled carbon nanotubes (CNTs) using NaBH4 as reducing reagent. The prepared catalyst was analyzed using X-ray diffraction pattern, field emission-scanning electron microscopy, energy-dispersive X-ray spectroscopy (EDS) and Brunauer–Emmett–Teller method. The differential scanning calorimetry (DSC) curves of Mg/KNO3 and Mg/KNO3/Ag/CNTs pyrotechnic were studied at heating rates of 5, 10, 15 and 20 °C min−1 under nitrogen atmosphere. The shift of the temperature of exothermic peak to lower temperatures and increasing of enthalpy of ignition reaction of Mg/KNO3 pyrotechnic was seen in DSC curves in presence of Ag/CNTs catalyst. The activation energies (Ea) of ignition reaction of Mg/KNO3 and Mg/KNO3/Ag/CNTs pyrotechnics were calculated 172–186 and 152–165 kJ mol−1, respectively, using the free-model methods of Kissinger, Ozawa–Flynn–Wall and Kissinger–Akahira–Sunose. Also the pre-exponential factor (A) and kinetic model function of ignition reaction of pyrotechnics were determined by the compensation effect and nonlinear model fitting method. The values of pre-exponential factor were obtained 2.9 × 1012 and 1.1 × 1010 min−1 for the ignition reaction of Mg/KNO3 in absence and in presence of catalyst of Ag/CNTs, respectively. The mechanism functions of Avrami–Erofeev A2\(\left( {f(a) = 2\left( {1 - \alpha } \right)\left[ { - { \ln }\left( {1 - \alpha } \right)} \right]^{1/2} } \right)\) and A3\(\left( {f\left( \alpha \right) = 3\left( {1 - \alpha } \right)\left[ { - \ln \left( {1 - \alpha } \right)} \right]^{2/3} } \right)\) were found to be the best pattern for ignition reaction of Mg/KNO3 and Mg/KNO3/Ag/CNTs pyrotechnics, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The result of ignition reaction of a pyrotechnic such as mixture of potassium nitrate and magnesium as oxidant and fuel, respectively, is production of light and sound [1]. Various factors affect the enthalpy of ignition reaction of the pyrotechnic compositions. These factors include the choice of fuel and oxidizer, fuel to oxidizer ratio, degree of mixing, particle size, particle shape and presence of additives. Among the additives, catalyst is the most important component. They are chemical agents that increase the rate of chemical reactions, normally without being consumed in the process [2].
Pyrotechnically, the catalysts reduce the activation energy of the burning process, typically by reducing the decomposition temperature of the oxidizer (i.e., the temperature at which oxygen is made available). The operation and performance of pyrotechnic such as the burning rate and light output are dependent to the type of catalyst and the mass percent of it in the pyrotechnic formulation [1,2,3].
The use of several zero-valent metals (Zn, Cu, Co, etc.), metal oxides (Fe2O3, CuO, MnO2, etc.) and other metal compounds (CoFe2O4, CuFe2O4, etc.) was reported as a catalyst in formulation of pyrotechnics contains ammonium perchlorate (AP) oxidizer [4,5,6].
Added nanocatalysts can improve its burning ability. Nanoparticles are very small, their specific surface is very large, and they are characterized by lots of reaction centers [7, 8]. But nanoparticles are prone to agglomeration, and they cannot be mixed with the other components of a formulation very easily. All these factors restrict their application. For remove of these restricts, the use of allotropes of carbon is proposed. As an allotropic form of carbon, CNTs show diameters in the nanometer range and a large specific surface, so CNTs can be considered to be a good substrate [4]. The metal nanoparticles cannot agglomerate due to the substrate of CNTs, so the catalytic performance of metal nanoparticles in the substrate of CNTs would be improved [9].
Carbon nanotubes (CNTs) were added into pyrotechnics with potassium perchlorate and potassium nitrate by water-mixing method and acetone-mixing method, respectively, and were due to increasing 4.15 and 1.51 times of reaction rate [10]. Cobalt nanoparticles in the substrate of carbon nanotubes (CNTs) were prepared by microwave-assisted method. The addition of 5 mass% Co/CNTs decreased the high decomposition temperature of AP by 174.05 °C and increased the total DTA heat release by 0.799 kJ g−1 [11].
Magnesium was used as metallic fuels in pyrotechnic compositions, and produces a very high light output. The compositions of Mg and an oxidizer such as potassium nitrate are used to make different type of flares, as well as tracer applications [12]. The oldest solid oxidizer that was used in high-energy mixtures was potassium nitrate (or saltpeter, as it was known for many years). Its advantages are ready availability of high-purity material at reasonable cost, low hygroscopicity, and the relative ease of ignition in many mixtures. The ignitability of it is related to the low (334 °C) melting point. It has a high (39.6%) active oxygen content, and decomposes at high temperature according to the equation \(2{\text{KNO}}_{3} = {\text{K}}_{2} {\text{O}} + {\text{N}}_{2} + 2.5{\text{O}}_{2}\). This reaction is a strongly endothermic reaction (ΔH = + 75.5 kcal mol−1 of KNO3). With good fuels (such as charcoal and active metals), potassium nitrate will react well. Potassium nitrate shows not explosion reaction, even when very strong initiating modes are used [1, 13].
Thermal analysis methods of TG and DSC are used for study of the ignition reaction of pyrotechnics. Ignition is the process of stimulating a pyrotechnic composition to release its internal energy and can be defined as “the initiation of self-sustained burning or explosion of a pyrotechnic material”. The ignition behavior of pyrotechnics depends on different factors, such as oxygen balance, thermal stability and the decomposition temperature of the oxidizer [14, 15].
The application of thermal analysis for study of pyrotechnics is a specialized field of activity. Even so, it has had a significant influence on our understanding of the mechanism of pyrotechnic reactions. The interpretation of thermal analysis curves for pyrotechnics must be careful. Under conditions which lead to ignition, a sharp exothermic peak is obtained in which the measured temperature rise may be several hundred degrees. Although the temperature of ignition may be obtained, the mechanism relates to the thermal analysis environment and is unlikely to be the same as that for ignition by a hot wire or fuse. The temperature thus obtained may vary significantly according to the experimental conditions. Where ignition does not occur, several peaks are usually obtained, and it is under these conditions that DTA and DSC have proved to be of particular value [16, 17].
The synthesis and characterization of Ag/CNTs catalyst is reported in this paper. The ignition reaction of pyrotechnic compositions of Mg/KNO3 and of Mg/KNO3/Ag/CNTs is also investigated using the TG/DSC curves of the thermal events. The kinetic triplet of Mg/KNO3 pyrotechnic is also reported in the absence and in the presence of Ag/CNTs catalyst.
Experimental
Materials
The multi-walled carbon nanotubes (MWCNTs) were purchased from Sigma-Aldrich Port Company. Silver nitrate (AgNO3), sodium citrate and NaBH4 were purchased from Merck Company and all the chemical reagents were analytical grade.
Analytical grade of Mg and KNO3 powders with purity > 99% was purchased from Merck Company. The salt of potassium nitrate was ground in a mortar to produce a fine powder and then dried at 80 °C for 2 h. The particles size distribution of Mg and KNO3 powders were determined using a laser particle size analyzer (FRITSCH, model Analystte 22, Micro Tec Plus). The median (D50) of particles size was obtained 63.5 and 59.1 µm, respectively, for powders of Mg and KNO3.
Catalyst preparation
The CNTs were purified by a sequence of steps, including circulation reflux by mixed acid of concentrated HNO3 and HCl at 100 °C for 2 h. The obtained CNTs were finally washed with distilled water to neutral pH, then dried [4, 11].
Ag nanoparticles were loaded on CNTs by growth method of them using NaBH4 as reductant [18]. The procedure is briefly introduced as following: 80 mg CNTs were added into 80 mL deionized water and ultrasonically dispersed homogeneously, followed by adding 2.5 mL of 0.1M AgNO3 and 2.5 mL of 0.1M sodium citrate solution. The resulting solution was quickly mixed by magnetic stirring vigorously in an ice/water bath. The reduction reaction was carried out by introducing 5.0 mL of 0.3M NaBH4 aqueous solution slowly to the above solution. After that, the solution was kept in the ice/water bath for 5 h and aged at room temperature for 25 h. Finally, the products were filtered, washed with deionized water and ethanol, and dried in a vacuum oven at 80 °C overnight before collection. The loading of Ag on CNTs was about 25 mass% that was confirmed using inductively coupled plasma (ICP)-atomic emission spectrometry.
Characterization of catalyst
FE-SEM image and EDS spectra of Ag/CNTs nanoparticles were obtained by a VEGA-Mira 3-XMU field emission—scanning electron microscope (FE-SEM). XRD (X-ray diffraction) patterns of catalyst were recorded by a Diffractometer Philips X. PERTPRO with anode of Cu (λ = 1.5406 Å of Cu Kα) and filter of Ni. BET method and N2 adsorption isotherms were used to estimation of surface area of nanopowders. The adsorption of nitrogen gas at 77 K by a Belsorp Mini Π instrument was used.
TG/DSC curves of pyrotechnic
The composition of Mg/KNO3 pyrotechnic was prepared 37:63 (w:w) by intimately mixing the powders in a mortar about 15 min. The synthesized Ag/CNTs nanoparticles was added to the mixture of Mg/KNO3 in a 5:95 mass ratio and the mixture was thoroughly mixed in the mortar for about 30 min.
TG/DSC measurements were carried out using a Perkin Elmer simultaneous thermal analyzer model STA 6000 (USA). Alpha-alumina vessels with 70 μL volume were used as sample containers and alumina powder was used as the reference material. The thermal analysis of samples was performed under nitrogen atmosphere with flow rate of 50 mL min−1. DSC curves were obtained at different heating rates of 5, 10, 15, and 20 °C min−1 from ambient temperature (25 °C) to 800 °C.
Results and discussion
Characterization of Ag/CNTs nanoparticles
XRD patterns of nanoparticles of Ag/CNTs are shown in Fig. 1. The broad diffraction peak with 2θ at 26.5 related to disordered CNTs. The diffraction peaks with 2θ at 38.8°, 43.5°, 65.5° and 78.2° that are related to reflections of Ag (111), (200), (220) and (311), respectively, indicate the formation of Ag nanocrystals with cubic face centered structure [19, 20]. This result shows that the Ag nanoparticles are well crystallized without any impurity. Because, no other impurity peaks are observed in XRD patterns. The nanoparticles size of the Ag crystalline is calculated from the major diffraction peak (111) (2θ = 38.8°) using Scherrer’s formula [21]. An average nanoparticle size is 27.8 nm.
FE-SEM image of prepared Ag/CNTs nanoparticles is presented in Fig. 2. The surface of CNTs is rough when supported with Ag nanoparticles. The formation of silver nanoparticles with dimension of less than 100 nm is confirmed by FE-SEM image. The results of EDS (Fig. 3) show that the matter on the surface of CNTs is C, Ag and O elements. The source of oxygen element is the H2O molecules and carboxyl group [22].
The specific surface area of MWCNTs and Ag/CNTs nanoparticles are calculated by the BET method. The obtained results indicated the surface area of 102.8 and 78.4 m2 g−1 for MWCNTs and Ag/CNTs nanoparticles, respectively. The decrease of surface area of the catalyst Ag/CNTs can be related to an increase in density of it in comparison with MWCNTs. Also, the filling of pores by metal nanoparticles can reduce the surface area [23].
The effect of catalyst on TG/DSC curves of Mg/KNO3 pyrotechnic
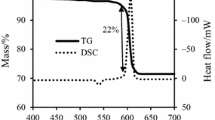
TG/DSC curves of Mg/KNO3 pyrotechnic at heating rate of 5 °C min−1 are shown in Fig. 4. By respect to the mole ratio of 5:2 of Mg to KNO3 (Eq. 1), the mass ratio of 37:63 was used for this pyrotechnic [1, 15].
As seen from Fig. 4, DSC curve shows an endothermic peak and an exothermic peak at temperatures 332.7 and 621.3 °C that are related to the melting of KNO3 and combustion reaction of Mg and potassium nitrate, respectively [24, 25]. Surface area of exothermic peak shows the enthalpy (ΔH) value of combustion reaction. The ΔH value is − 4878.5 J g−1. Additionally, a mass reduction is observed with initial temperature of 600.5 °C in TG curve. The Mg content of this pyrotechnic sample is reacted and the mass gain is not observed after combustion reaction [15, 26].
TG/DSC curves of Mg/KNO3 pyrotechnic in the presence of nanoparticles of Ag, CNTs and Ag/CNTs as catalyst are shown in Fig. 5a–c, respectively. The mass ratio of used catalyst in the mixture was 5 mass%. Compared to Fig. 4, the peak temperature and enthalpy of combustion reaction are changed. Tmax values are seen at temperatures 603.2, 596.8, and 584.2 °C in Figs. 5a–c, respectively. Additionally, the combustion reaction of Mg/KNO3 pyrotechnic shows the enthalpy (ΔH) values − 4937.5, − 5114.4 and − 5375.7 J g−1, respectively, in presence of nanoparticles of Ag, CNTs and Ag/CNTs, respectively. The presence of nanocatalysts led to a shift in Tmax of exothermic peak and an increase in enthalpy of combustion reaction [27]. In presence of Ag/CNTs nanoparticles as catalyst, the combustion reaction of Mg/KNO3 pyrotechnic is done in the lower temperature and with higher enthalpy. So that, a reduction 37 °C and an increase 500 J g−1 are seen for ignition reaction of Mg/KNO3 pyrotechnic in presence of Ag/CNTs nanocatalyst [28, 29]. The combustion of CNTs was seen in temperature of 580–600 °C. Due to the fact that the combustion reaction of pyrotechnic is seen at this range of temperature, the combustion reaction of CNTs is not seen separately in DSC curves. Additionally, low catalyst amounts lead to modest changes in the enthalpy of the pyrotechnic combustion reaction [30]. However, the catalyst of Ag/CNTs is due to the improvement of combustion reaction of Mg/KNO3 pyrotechnic. Metal nanoparticles such as silver nanoparticles cannot agglomerate due to the support of CNTs. So the catalytic performance of metal nanoparticles is supported on CNTs [18, 31].
Kinetic parameters of ignition reaction
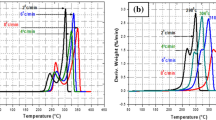
DSC curves showed that the presence of proposed catalyst of Ag/CNTs is due to a reduction of temperature and increasing of enthalpy of ignition reaction of Mg/KNO3 pyrotechnic. The kinetic of ignition reaction of pyrotechnic sample was studied using DSC curves of Mg/KNO3 and Mg/KNO3/Ag/CNTs at different heating rates of 5, 10, 15, and 20 °C min−1 under nitrogen atmosphere (Figs. 6 and 7).
The exothermic peak of the ignition reaction of Mg/KNO3 was observed at temperatures of 621.8, 646.3, 662.3 and 673.8 °C, respectively, at heating rates of 5, 10, 15 and 20 °C min−1 (Fig. 6). While this peak is seen at temperatures of 584.2, 611.1, 627.1 and 641.3 °C, respectively, at heating rates of 5, 10, 15 and 20 °C min−1 for Mg/KNO3/Ag/CNTs sample (Fig. 7). Additionally, these results show that the area of exothermic peak is decreased with increasing the heating rate [32].
Equation (2) shows that an ignition reaction similar to any thermal process can be investigate using a function of two variables, temperature (T) and extent of conversion (α) [33].
where A and Ea are kinetic parameters, the Arrhenius constant and the activation energy, respectively, R is the universal gas constant and f(α) is the kinetic model of the reaction in a differential form. The α value (from 0 to 1) is determined experimentally as a fraction of the change in mass and or as a fraction of the total heat released or absorbed in the process [33, 34].
The temperature is controlled as isothermal, T = constant and non-isothermal, T = T(t). The temperature changes linearly with time in nonisothermal method with the heating rate of β = dT/dt = constant.
The several methods such as Kissinger [35], Ozawa–Flynn–Wall (OFW) [36] and Kissinger–Akahira–Sunose (KAS) [37] can be used for determination of kinetic parameters of ignition reaction of pyrotechnic. The Eqs. (3), (4) and (5), respectively, indicate the Kissinger, OFW and KAS methods.
where g(α) is the integral form of the reaction model of f(α). On the basis of the Eqs. (3), (4) and (5), Ea values are obtained from the slope of plot \({ \ln }\left( {\beta /T_{\text{m}}^{2} } \right){\text{ versus }}1 /T_{\text{m}}\), plot of log(β) versus 1/T, and plot \({ \ln }(\beta /T_{\text{a}}^{1.92} ){\text{ versus }}1 /T_{\text{a}}\), respectively [33]. The Ea values are collect in Table 1.
The study the thermal behavior of energetic materials is important for their safe processing, handling and storage. As seen from Table 1, Ea value calculated by Kissinger’s method is in rough agreement with that by OFW’s method. Also, the average of Ea values of KAS’s method is close to the results of two methods of Kissinger and OFW. KAS results show uniformity in Ea values for α value of 0.1–0.9, therefore, a single-step mechanism is proposed for ignition reaction of pyrotechnic [38].
As mentioned, the reaction model, f(α), along with Ea value and Arrhenius coefficient are triplet kinetic. The reaction model, Arrhenius coefficient and activation energy are named the “kinetic triplet” [33]. The conversion–temperature (α–T) curves at heating rates of 5, 10, 15 and 20 °C min−1 for thermal ignition reaction were used for prediction of reaction model. Avrami–Erofeev, Mampel power law, Power law, Contracting and Dimensional diffusion are models that are used to predict the reaction path in thermal process [33]. The curve of α versus T is used to predict the reaction model. Curves of α–T for ignition reaction at different heating rates are shown in Fig. 8a, b for pyrotechnics of Mg/KNO3 and Mg/KNO3/Ag/CNTs, respectively. As seen, the α–T curves indicate a sigmoidal shape. Therefore, Avrami–Erofeev functions are used for selection of a proper mechanism function for ignition reaction of Mg/KNO3 and Mg/KNO3/Ag/CNTs pyrotechnics.
The model fitting method for differential equation (Eq. 6) is used for prediction the best function f(α) [33, 36].
According to the above mentioned equation, the plots of ln[(dα/dT)/f(α)(Ea(T − To)/RT2 + 1)] versus 1/T at different heating rates (β) can be obtained by linear regression [33]. The most probable mechanism function f(α) is the function that affords the most linear plot with a linear regression coefficient R2 near 1.000.
The compensation effect [33] was used for obtaining the best reaction model and pre-exponential factor. The calculated Ei and Ai values from each of the models at different heating rates were replaced into Eq. (7) to determine the compensation effect parameters a and b.
The pre-exponential factor Ao was calculated by substitution of the calculated Eo from the model free method and a and b parameters in Eq. (8):
The calculated results are presented in Table 2. Eo and Ao values were substituted into Eq. (9).
The calculated numerical values of f(α) were compared with the theoretical dependencies obtained from f(α) equations to identify the best matching model. By applying this method to all of the reaction models, the mechanism functions of Avrami–Erofeev A2 (f(α) = 2(1 − α)[− ln(1− α)]1/2) and Avrami–Erofeev A3 (f(α) = 3(1 − α)[− ln(1 − α)]2/3) were found to be the best pattern for ignition reaction of Mg/KNO3 and Mg/KNO3/Ag/CNTs pyrotechnics, respectively. The plots of the theoretical and experimental f(α) versus α at different heating rates for ignition reaction is presented in Fig. 9. By means of nonlinear regression method [39, 40], the difference between theoretical and experimental f(α) was calculated through the residual sum of square (RSS) that should be minimum according to Eq. (10):
The data of Tables 1 and 2 show the harmony in activation energy values of free-model methods and model fitting method. RSS values in Table 2 indicate the fitting of theoretical and experiments amounts of f(α). However, RSS data of predicted model of Mg/KNO3 pyrotechnic show the more consistency of f(α) values.
Conclusions
The nanoparticles of Ag/CNTs show the catalytic effect on the ignition reaction of Mg/KNO3 pyrotechnic. The exothermic peak of ignition reaction of pyrotechnic is seen a decrease 37 °C in Tmax and an increase 500 J g−1 in enthalpy in presence of Ag/CNTs nanocatalyst using DSC curves. Additionally, the activation energy of combustion reaction of Mg/KNO3 pyrotechnic is calculated 155 kJ mol−1 in presence of the catalyst. While, Ea value is 174 kJ mol−1 in absence of Ag/CNTs.
The model functions of A2 (f(α) = 2(1 − α)[− ln(1 − α)]1/2) and A3 (f(α) = 3(1 − α)[− ln(1 − α)]2/3) were the predicted models as mechanism of ignition reaction of Mg/KNO3 pyrotechnic in absence and in presence of Ag/CNTs nanocatalyst using the compensation effect.
References
Conkling JA, Mocella C. Chemistry of pyrotechnics basic principles and theory. 2nd ed. London: Taylor and Francis; 2010.
McLain JH. Pyrotechnics, from the viewpoint of solid state chemistry. New York: Franklin Institute Press; 1980.
Kosanke KL, Kosanke BJ. Parallel and propagative burning. Pyrotechnics Guild Int Bull. 1992;79:167–72.
Ping C, Li F, Jian Z, Wei J. Preparation of Cu/CNT composite particles and catalytic performance on thermal decomposition of ammonium perchlorate. Propellants Explos Pyrotech. 2006;31:452–5.
Liu LL, Li FS, Tan LH, Ming L, Yi Y. Effects of nanometer Ni, Cu, Al and NiCu powders on the thermal decomposition of ammonium perchlorate. Propellants Explos Pyrotech. 2004;29:34–8.
Zhao S, Ma D. Preparation of CoFe2O4 nanocrystallites by solvothermal process and its catalytic activity on the thermal decomposition of ammonium perchlorate. J Nanomater. 2010;201:1–5.
Pouretedal HR, Shevidi O, Nasiri M, Pourhasan FS. Red water treatment by photodegradation process in presence of modified TiO2 nanoparticles and validation of treatment efficiency by MLR technique. J Iran Chem Soc. 2016;13:2267–74.
Pouretedal HR, Ravanbod M. The catalytic effect of Mn2O3 nanoparticles on the ignition reaction of pyrotechnic of ammonium nitrate(V)/thiourea. Cent Eur J Energy Mater. 2017;14:430–47.
Ma S, Luo R, Gold JI, Yu AZ, Kim B, Kenis PJA. Carbon nanotube containing Ag catalyst layers for efficient and selective reduction of carbon dioxide. J Mater Chem A. 2016;4:8573–8.
Xin-ming Q, Nan D, Si-fan W, Zeng-yi L. Catalytic effect of carbon nanotubes on pyrotechnics. Chin J Energ Mater. 2009;17:603–7.
Zhang X, Jiang W, Song D, Liu Y, Geng J, Li F. Preparation and catalytic activity of Co/CNTs nanocomposites via microwave irradiation. Propellants Explos Pyrotech. 2009;34:151–4.
Lăzăroaie C, Eşanu S, Său C, Petre R, Iordache PZ, Staikos G. Temperature measurements of magnesium- and aluminium-based flares. J Therm Anal Calorim. 2014;115:1407–15.
Shidlovskiy AA: Principles of pyrotechnics, 3rd ed., Moscow, 1964. (Translated by Foreign Technology Division, Wright-Patterson Air Force Base, OH, 1974).
Babar Z, Malik AQ. Thermal decomposition, ignition and kinetic evaluation of magnesium and aluminium fuelled pyrotechnic compositions. Cent Eur J Energy Mater. 2015;12:579–92.
Pouretedal HR, Ravanbod M. Kinetic study of ignition of Mg/NaNO3 pyrotechnic using non-isothermal TG/DSC technique. J Therm Anal Calorim. 2015;119:2281–8.
Laye PG, Charsley EL. Thermal analysis of pyrotechnics. Thermochim Acta. 1987;120:325–49.
Pourmortazavi SM, Hosseini SG, Hajimirsadeghi SS, Fareghi Alamdari R. Investigation on thermal analysis of binary zirconium/oxidant pyrotechnic systems. Combust Sci Technol. 2008;180:2093–102.
Yang G-W, Gao G-Y, Wang C, Xu C-L, Li H-L. Controllable deposition of Ag nanoparticles on carbon nanotubes as a catalyst for hydrazine oxidation. Carbon. 2008;46:747–52.
Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6:399–408.
Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. Leaves and their synergistic effects with antibiotics. J Radiation Res Appl Sci. 2016;9:217–27.
Pouretedal HR, Tofangsazi Z, Keshavarz MH. Photocatalytic activity of mixture of ZrO2/SnO2, ZrO2/CeO2 and SnO2/CeO2 nanoparticles. J Alloys Compounds. 2012;513:359–64.
Yamada T, Hayashi Y, Takizawa H. Synthesis of carbon nanotube/silver nanocomposites by ultrasonication. Mater Trans. 2010;51:1769–72.
Zl L, Xy L, Xd S, Jy L. Carbon-supported Pt and PtRu nanoparticles as catalysts for a direct methanol fuel cell. J Phys Chem B. 2004;108:8234–40.
Zhu CG, Wang HZ, Min L. Ignition temperature of magnesium powder and pyrotechnic composition. J Energ Mater. 2014;32:219–26.
Brown SD, Charsley EL, Goodall SJ, Laye PG, Rooney JJ, Griffiths TT. Studies on the ageing of a magnesium–potassium nitrate pyrotechnic composition using isothermal heat flow calorimetry and thermal analysis techniques. Thermochim Acta. 2003;401:53–61.
Pouretedal HR, Loh Mousavi S. Study of the ratio of fuel to oxidant on the kinetic of ignition reaction of Mg/Ba(NO3)2 and Mg/Sr(NO3)2 pyrotechnics by non-isothermal TG/DSC technique. J Therm Anal Calorim. 2018;132:1307–15.
Ting A, Hui-Qun C, Feng-Qi Z, Xiao-Ning R, De-Yu T, Si-Yu X, Hong-Xu G, Yi T, Li-Bai X. Preparation and characterization of Ag/CNTs nanocomposite and its effect on thermal decomposition of cyclotrimethylene trinitramine. Acta Phys Chim Sin. 2012;28:2202–8.
Dave PN, Vara J. Nano metal oxide as Potential catalyst for thermal decomposition of Ammonium nitrate. Int J Res. 2017;4:943–6.
Xu Z-X, Xu G-S, Fu X-Q, Wang Q. The mechanism of nano-CuO and CuFe2O4 catalyzed thermal decomposition of ammonium nitrate. Nanomater Nanotechnol. 2016;6:1–10.
Pouretedal HR, Basati S. Characterization and Photocatalytic Activity of ZnO, ZnS, ZnO/ZnS, CdO, CdS and CdO/CdS Nanoparticles in Mesoporous SBA-15. Iran J Chem Chem Eng. 2015;34:11–9.
Liu Y, Wu G, Cui Y. Ag/CNT-catalyzed hydroamination of activated alkynes with aromatic amines. Appl Organomet Chem. 2013;27:206–8.
Sinapour H, Damiri S, Pouretedal HR. The study of RDX impurity and wax effects on the thermal decomposition kinetics of HMX explosive using DSC/TG and accelerated aging methods. J Therm Anal Calorim. 2017;129:271–792.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Ravanbod M, Pouretedal HR, Amini MK, Ebadpour R. Kinetic study of the thermal decomposition of potassium chlorate using the non-isothermal TG/DSC technique. Cent Eur J Energy Mater. 2016;13:261–70.
Liu J, Song D, Guan H. Isothermal kinetics approach to investigating the oxidation process of red phosphorus in air. J Therm Anal Calorim. 2017;128:1801–10.
Vyazovkin S, Wight CA. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int Rev Phys Chem. 1998;17:407–33.
Pouretedal HR, Damiri S, Ghaemi EF. Non-isothermal studies on the thermal decomposition of C4 Explosive using the TG/DTA Technique. Cent Eur J Energ Mater. 2014;11:285–94.
Mohamed MA, Attia AK. Thermal behavior and decomposition kinetics of cinnarizine under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2017;127:1751–6.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Pouretedal HR, Ebadpour R. Application of non-isothermal thermogravimetric method to interpret the decomposition kinetics of NaNO3, KNO3, and KClO4. Int J Thermophys. 2014;35:942–51.
Acknowledgements
We would like to thank the research committee of Malek-ashtar University of Technology (MUT) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouretedal, H.R., Shahmoradi, M., Zareh, A. et al. The catalytic effect of Ag/CNTs nanocomposite on the ignition reaction of Mg/KNO3 pyrotechnic by determination of the kinetic triplet. J Therm Anal Calorim 135, 2975–2983 (2019). https://doi.org/10.1007/s10973-018-7554-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7554-7