Abstract
Sigma phase precipitation occurring during the exposure of duplex stainless steels in the temperature range from 800 to 900 °C deeply affects the material toughness and corrosion resistance. σ-Phase precipitation process is strongly influenced by many physical parameters, such as the specific chemical composition, the ferrite amount and its average grain size, and the entity of plastic deformation due to the previous technological process. The strong dependencies of σ-phase precipitation on all these factors justify the continuous study of the process kinetics. This paper focuses on the σ-phase precipitation kinetics in F55 steel grade. The investigation has been performed by an innovative experimental method, such as the anisothermal dilatometric technique. The application of the Kissinger’s method has been used for deriving the process activation energy and kinetics. The results have been compared with the ones obtained by metallographic analysis and hardness tests performed on isothermally aged samples, heat-treated in a laboratory furnace at 850 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Austeno-ferritic or duplex stainless steels are characterized by biphasic microstructure at room temperature. The coexistence of austenite and ferrite confers the material an excellent combination of mechanical and corrosion resistance, which allows the application of these alloys in harsh environment under high loads, even at low temperatures. Therefore, common application fields are the Oil & Gas and chemical industries and the offshore and desalination plants.
The material chemical composition and the temperature of the solution treatment are the two main factors affecting the amount of austenite and ferrite at room temperature. For a given chemical composition, the heat treatment is the key for getting the desired material properties, especially in terms of low temperature toughness [1]. Setting the proper solution temperature and time is not enough for granting a good result because the cooling rate is fundamental to avoid the precipitation phenomena this material is very prone to. Any soaking in the temperature range from 1000 to 300 °C might favour the precipitation of secondary phases deleterious for the material properties, in terms of both mechanical properties, mainly impact strength, and corrosion resistance [1,2,3].
The soaking in the upper temperature range favours σ-phase precipitation, according to the following eutectoid reaction:
indicating that the ferrite transforms into a lamellar structure of sigma and gamma [1]. Sigma phase precipitation mainly occurs at the intersection of three grains and at the grain boundaries between ferrite and austenite, growing towards ferrite and reaching the maximum rate in the temperature range from 800 to 850 °C [1, 2]. The detrimental effect of σ-phase on the material toughness is well-known: even a small amount causes an important loss of the impact and corrosion resistance [3].
The need of further investigating σ-phase precipitation rises from the fact that the precipitation process is strongly influenced by many physical parameters, such as the specific chemical composition, the ferrite amount and the average grain size, and the entity of plastic deformation due to the previous technological process [4].
The most common techniques used for the investigation of σ-phase precipitation are metallographic analysis and hardness tests carried out on as-solutioned samples submitted to isothermal treatments in the critical temperature range [1,2,3].
In this paper the precipitation phenomena of F55 steel grade have been investigated by an innovative experimental method, such as the dilatometric technique. In the technical literature, it is known that dilatometry is a useful technique for studying the microstructural transformations by measuring the dimensional variation of samples at constant temperature [5,6,7,8,9] and allowing the calculation of the activation energy of the involved phenomena by continuous heating [10,11,12]. As the precipitation of sigma phase is known to be very rapid in a given temperature range, in the present work the dilatometric analysis during continuous heating was selected as an innovative experimental method for studying the precipitation phenomena of F55 steel grade. The technical literature also states the efficacy of this method, which results well agree with the ones obtained by differential thermal analysis (DTA) and differential scanning calorimetry (DSC) [13] or are considered even more reliable [14].
The obtained results have been compared with the data obtained by traditional experimental techniques, such as microstructural analysis and hardness measurements.
Materials and methods
The investigated material is an ASTM A182/A182M-16 F55 [15] steel grade supplied as a bar of 660 mm diameter. The chemical composition of the bar is reported in Table 1.
The bar was forged and heat-treated in an industrial plant.
A transversal section of the forged bar has been machined, and some blocks cut from the section. The blocks have been solution annealed in a laboratory furnace at 1120 °C, 1 h/inch, according to ASTM A182/A182M-16.
Anisothermal dilatometric tests have been performed on as-solutioned samples (10 × 10 × 55 mm3). All the specimens have been positioned in a quartz sample holder in a NETZSCH DIL402 ES dilatometer and heated from room temperature (Troom) to 900 °C, with different heating rates (2.5, 5.0, 7.5 K min−1). All the tests have been performed in a N2 + 5%H2 atmosphere. The variation of the sample length versus time has been measured and continuously recorded. The specimens’ microstructure has been investigated at the end of each test by LOM (light optical microscope) and SEM (scanning electron microscope).
A Leica Light Optical Microscope with colours digital camera and a Zeiss EVO 50XVP Scanning Electron Microscope with X-ray EDS microanalysis and Electron Backscattered Diffraction System have been used.
Isothermal ageing treatments have been performed on small samples (10 × 10 × 20 mm3) at 850 °C in a laboratory furnace at different times. The value of the precipitation temperature (850 °C) was selected for reproducing a very fast kinetics according to the precipitation curve available in technical literature [3, 16]. The maximum soaking time was 60 min, and all the samples have been water quenched.
The metallographic analysis by LOM and SEM and hardness tests have been performed on the solution annealed material and after the isothermal treatments.
The metallographic samples have been mirror-polished following the standard metallographic techniques and electrolytically etched with 10% oxalic acid solution. The etching parameters have been selected according to the standard ASTM E407-07 [17]. The austenite/ferrite (A/F) ratio and the amount of precipitated “σ + γ” phase have been estimated by image analysis techniques implemented in the software Image Pro Plus. The estimation of “σ + γ” phase amount has been done by averaging the results on three images per sample, at low magnification (50×).
Hardness tests have been performed for characterizing all the obtained conditions. Vickers macro- and microhardness tests have been done for getting, respectively, the material and the ferrite hardness. For each sample, from 3 to 5 hardness tests have been performed according to UNI EN ISO 6507 standard and the results have been averaged [18].
Results
The solution treatment performed on the forged material resulted in a A/F ratio of about 1:1, and no secondary phases were detected, as shown in Fig. 1. The material properties after the solution annealing treatment are summarized in Table 2.
Dilatometric tests
From the recorded data, the sample expansion curve and its derivative have been obtained. Figure 2 shows an example of dilatometric curve obtained with 2.5 K min−1 heating rate, together with its first derivative curve, which is characterized by the presence of two negative peaks in the temperature range from 700 to 900 °C.
Figure 3 shows the first derivative curves for each experimented heating rate. In agreement with some results reported in the technical literature [9], as the heating rate increases, the identified negative peaks are placed at higher temperatures.
As known in literature [10], the Kissinger method can be used for deriving activation energies from temperature-scanned experiments. It is based on the fact that the observed temperature of a peak (Tp) depends on the scan rate (S = dT/dt) of the experiments. The method has been generalized and justified for solid-state reactions by Mittemeijer et al. [19] The Kissinger expression, as modified by Mittemeijer et al., relates Tp to S as follows:
where Tp = peak temperature (K); S = heating rate (K s−1); Eact = effective activation energy for the process associated with the peak (J mol−1); R = gas constant (8.314 J mol−1 K−1); k0 = pre-exponential factor in the Arrhenius equation for the rate constant k:
The anisothermal tests data, for heating rate from 2.5 to 7.5 K min−1, have been analysed by the Kissinger method to yield activation energies (Eact) and rate constants (k0).
The presence of two negative peaks in the temperature range from 700 to 900 °C indicates that two different transformations occurred. According to technical literature [2], the first peak is associated with the precipitation of chromium compounds, while the second peak is associated with the formation of sigma phase.
As Fig. 3 shows, a peak temperature is associated to each scan rate for both the transformations. By associating the corresponding peak temperature to its scan rate according to Eq. (2), the activation energies of the two precipitation phenomena have been calculated.
For both the transformations, the identified peak temperatures corresponding to each heating rate and the obtained activation energies are reported in Table 3, together with the values of k0 useful for evaluating the process kinetics.
In agreement to the dilatometry tests results, the metallographic analysis revealed the occurrence of two different precipitation phenomena. In fact, as shown in Fig. 4, the ferritic matrix has been completely substituted by the eutectoid “σ + γ” and chromium-based precipitates (in correspondence of the arrows) are clearly visible in the austenitic regions.
EDXS analysis has been performed by SEM. The results obtained by the analysis of the sample heated at 2.5 K min−1 are reported in Fig. 5 and Table 4, confirming the presence of σ-lamellae (position A) and γ-phase (position B).
Finally, using the activation energy and k0 value obtained by analysing the second transformation peaks, which are the ones related to σ-phase precipitation, the kinetics of σ-phase precipitation at 850 °C has been calculated according to Eq. (3) and the obtained k value is 1.10 × 10−3.
Metallographic analysis
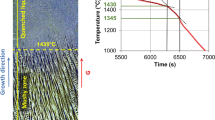
Figure 6 shows the evolution in time of “σ + γ” phase precipitation during the soaking at 850 °C.
The SEM analysis confirmed the presence of σ-phase. The measured chemical composition (Table 5) in point A (Fig. 7) by EDXS analysis is compliant with the nominal chemical composition of σ-phase, so confirming the nature of the phase.
By an image analysis software, the fraction of “σ + γ” phase over ferrite was measured versus time and the results are shown in Fig. 8. In isothermal condition at 850 °C, the precipitation of σ-phase starts within 10 min and the transformation of the ferrite is almost completed after 1 h soaking. In fact, for the longest investigated time, the ferrite has been completely substituted by the eutectoid.
Hardness tests
The effect of σ-phase precipitation is also revealed by an increase of the material hardness, as shown in Fig. 9. For a soaking time of 60 min, the hardness increment with respect to the initial one is about 150 HV30. The hardness profile confirms that σ-phase nucleation starts after a soaking time of 10–15 min.
σ-phase precipitation kinetics by metallographic and hardness data
As reported by Magnabosco [20], the fraction of σ-phase can be described by a Johnson–Mehl–Avrami (JMA) type expression:
where X = fraction of σ-phase (0 < X < 1) formed during an isothermal treatment at a time period t evaluated from the beginning of the transformation (t0); k = rate constant defined by Eq. (3); n = JMA exponent.
The process fraction of the phenomenon has been calculated according to Eqs. (5) and (6):
where X(σ+γ) = process fraction estimated by the amount of “σ + γ” phase, Eq. (5); %(σ + γ)0 = amount of “σ + γ” detected after 10 min soaking at 850 °C; %(σ + γ)inf = amount of “σ + γ” detected after 60 min soaking at 850 °C; XHV = process fraction estimated by the hardness increase, Eq. (6); HV0 = hardness of the sample heat-treated for 10 min at 850 °C; HVinf = hardness of the sample heat-treated for 60 min at 850 °C.
The process fraction versus time obtained analysing the metallographic and the hardness data are shown in Fig. 10.
By plotting the logarithmic form of Eq. (4), the process kinetics has been evaluated knowing the process fraction at different times. The k values obtained by metallographic and hardness methods are reported in Table 6, together with the JMA exponent n.
Discussion
The calculation of the activation energies for the detected transformations at the temperatures around 800 and 850 °C are 280 and 201 kJ mol−1, respectively.
The latter activation energy of 201 kJ mol−1 is the one related to σ-phase formation, which has its maximum nucleation rate at 850 °C, and the subsequent transformation of δ-ferrite into “σ + γ” eutectoid. The obtained value agrees with the ones reported in the technical literature for σ-phase transformation. Blachowsky et al. [21] determined the activation energy with various method for a Fe53,8–Cr46,2 alloy, and it varies between 197 and 260 kJ mol−1. Ferro [22] determined 202 kJ mol−1 activation energy in 2205 duplex stainless steel through a semi-empirical model. The activation energy for the formation of σ-phase was determined 189 kJ mol−1 for a strongly deformed equiatomic Fe–V alloy by Costa et al. [23]. Finally, Berecz et al. [4] studied the decomposition kinetics of ferrite in isothermally aged SAF 2507-type duplex stainless steel by differential thermal analysis and estimated an activation energy for σ-phase of 243 kJ mol−1. The reported results clearly show the strong dependency of the values of activation energy on the material chemical composition and microstructural characteristics.
Moreover, the obtained results seem to indicate that the diffusion of Cr is reasonably the main thermally activated phenomenon for the nucleation of σ-phase. In fact, σ-phase nucleates at the intersection of three ferritic grains and at the grain boundaries between ferrite and austenite, growing towards the ferrite and the activation energies of Cr diffusion in the ferrite and for grain boundary diffusion are reported to be 241 and 218 kJ mol−1 [24], respectively.
The technical literature reports also an activation energy for Cr diffusion in the austenitic phase of about 293 kJ mol−1 [25]. This value is comparable with the estimated activation energy for the first detected transformation (280 kJ mol−1), suggesting that the presence of precipitates in the austenitic regions (indicated by the arrows in Fig. 3) is compatible with the formation Cr-rich compounds, which forms in the temperature range 700–900 °C.
The calculated σ-phase rate constant at 850 °C by dilatometric test is 1.10 × 10−3. The ones calculated by metallographic analysis and hardness tests are 2.83 × 10−4 and 3.07 × 10−4, respectively. Comparing all the obtained values, it is possible to observe that the process kinetics calculated by dilatometric data is faster than the one obtained by the traditional investigation methods. These results can be attributed to a different material condition prior to σ-phase precipitation during the anisothermal dilatometric tests. In fact, the set heating rate for dilatometric tests is sufficiently slow to favour base material modifications (mainly atoms diffusions), as confirmed by the precipitation of Cr-rich compounds, which could have accelerated the kinetics of σ-phase precipitation. [26]
Finally, the calculation of n, the JMA exponent, results in the value of 1165 (by metallography) and 1175 (by hardness tests), confirming that the δ-ferrite transformation into σ-phase is mainly a diffusion-controlled process, being n value between 0.5 and 2.5 [18].
Conclusions
In this paper, the precipitation phenomena of F55 steel grade in the temperature range from 700 to 900 °C have been investigated by an innovative experimental method, such as the dilatometric technique. The application of Kissinger method on the data obtained by anisothermal dilatometric tests resulted useful for the calculation of the activation energy and the kinetics of σ-phase precipitation. The obtained results have been compared with the ones obtained by traditional investigation methods, like metallography and hardness tests.
The performed analysis allows to conclude that:
-
the derivative of the dimensional change with respect to temperature allows to detect the temperature range of the precipitation
-
the effect of increasing the heating rate is to shift the precipitation to higher temperatures
-
the analysis of the non-isothermal dilatometric curves allows to estimate the apparent activation energy of σ-phase precipitation, which is in good agreement with the values reported by other authors;
-
the kinetics calculated by dilatometry appears faster than the values obtained by metallography and hardness data. This can be attributed to the precipitation of Cr-rich compounds anticipating the σ-phase formation.
-
metallographic analysis and hardness tests confirm to be equivalent methods for evaluating σ-phase precipitation, giving back comparable n and k values.
References
Nilsson JO. Overview: super duplex stainless steels. Mater Sci Eng. 1992;8:685–700.
Topolska S, Labanowsky J. Effect of microstructure on impact toughness of duplex and superduplex stainless steels. J Achiev Mater Manuf Eng. 2009;36:142–9.
Dominguez-Aguilar MA, Newman RC. Detection of deleterious phases in duplex stainless steels by weak galvanostatic polarization in alkaline solution. Corros Sci. 2006;48:2560–76.
Berecz T, Fazakas E, Mészáros I, Sajó I. Decomposition kinetics of ferrite in isothermally aged SAF 2507-type duplex stainless steel. J Mater Eng Perform. 2015. https://doi.org/10.1007/s11665-015-1793-6.
Rivolta B, Pinasco MR. Study of the age-hardening in an Au–Cu–Ag dental alloy with Pt and Pd additions with dilatometry, hardness measurements and microstructural analysis. Int J Microstruct Mater Prop. 2009;4:487–506.
Rivolta B, Gerosa R, Pinasco MR, Pellati G. Dilatometric analysis for investigating age hardening in a commercial precious metal dental alloy. J ASTM Int. 2009;6:1–12.
Gerosa R, Rivolta B, Derudi U. Dilatometric analysis to study aging of aluminum alloys. In: Canale LF, Narazaki M, editors. Quenching and cooling, residual stress and distortion control. ASTM Edition. p. 1055–65. ISBN: 978-0-8031-7509-9.
Gerosa R, Rivolta B, Sala A. Optimization of the heat treatment of a 17-4 PH stainless steel by dilatometric technique. In: Canale LF, Narazaki M, editors. Quenching and Cooling, Residual Stress and Distortion Control. ASTM Edition. p. 1066–76. ISBN: 978-0-8031-7509-9.
Rivolta B, Gerosa R. On the non-isothermal precipitation of copper-rich phase in 17-4 PH stainless steel using dilatometric techniques. J Therm Anal Calorim. 2010. https://doi.org/10.1007/s10973-010-0882-x.
Smith GW. Precipitation kinetics in an air-cooled aluminium alloy: a comparison of scanning and isothermal calorimetry measurements methods. Termochimica Acta. 1998;313:27–36.
Smith GW. Precipitation kinetics in solutionized aluminium alloy 2124: II. Effect of prior Guinier–Preston zone formation. Termochimica Acta. 1998;323:123–30.
Smith GW. Precipitation kinetics in solutionized aluminium alloy 2124: determination by scanning and isothermal calorimetry. Termochimica Acta. 1998;317:7–23.
Gojić M, Sućeska M, Rajić M. Thermal analysis of low alloy Cr–Mo steel. J Therm Anal Calorim. 2004;75:947–56.
Wielgosz E, Kargul T. Differential scanning calorimetry study of peritectic steel grades. J Therm Anal Calorim. 2015;119:1547–53. https://doi.org/10.1007/s10973-014-4302-5.
Standard specification for forged or rolled alloy and stainless steel pipe flanges, forged fittings, and valves and parts for high-temperature service, A182/A182M, ASTM International; 2016.
Hardin RA, Beckermann C. Simulations of the effect of section size and cooling on sigma phase formation in duplex stainless steel. In: Proceedings of the 66th SFSA technical and operating conference, Steel Founders’ Society of America; 2012.
Standard practice for microetching metals and alloys, E407, ASTM International; 2007.
Metallic materials—Vickers hardness test, UNI EN ISO 6507, UNI Standard; 2005.
Mittemeijer EJ, Cheng L, van der Schaaf PJ, Brakman CM, Korevaar BM. Analysis of nonisothermal transformation kinetics; tempering of iron-carbon and iron-nitrogen martensites. Met Trans. 1988;19A:925–32. https://doi.org/10.1007/BF02628377.
Magnabosco R. Kinetics of sigma phase formation in a duplex stainless steel. Mater Res. 2009. https://doi.org/10.1590/S1516-14392009000300012.
Blachoswski A, Dubiel SM, Zukrowski J. On the activation energy of the σ-phase formation in a pure and Ti-doped Fe-Cr alloy. Intermetallics. 2001;9:493–8.
Ferro P. A dissolution kinetics model and its application to duplex stainless steels. Acta Mater. 2013;61:3141–7.
Costa BFO, Cieslak J, Dubiel SM. Kinetics of σ-phase formation in equiatomic cold-rolled Fe–V alloys. Mater Chem Phys. 2013;143:19–25.
Bowen W, Leak GM. Diffusion in BCC iron base alloys. Metall Trans. 1970;1:2767–73.
Bowen W, Leak GM. Solute diffusion in alpha- and gamma-iron. Metall Trans. 1970;1:1695–700.
Lee KM, Cho HS, Choi DC. Effect of isothermal treatment of SAF 2205 duplex stainless steel on migration of δ/γ interface boundary and growth of austenite. J Alloys Compd. 1999;285:156–61.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rivolta, B., Gerosa, R. & Tavasci, F. The dilatometric technique for studying sigma phase precipitation kinetics in F55 steel grade. J Therm Anal Calorim 132, 869–877 (2018). https://doi.org/10.1007/s10973-017-6940-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6940-x