Abstract
The non-isothermal curing kinetics of epoxy resin system consisting of diglycidyl ether of bisphenol A (DGEBA) and m-xylylenediamine (MXDA) were investigated by high-pressure differential scanning calorimetry at different heating rates of 5, 10, 15 and 20 K min−1 under compressed carbon dioxide (CO2) of 0.1–8 MPa. The activation energies were determined by isoconversional Kissinger–Akahira–Sunose method and Vyazovkin numerical method, and the average values decreased from 53.5 to 46.8 and 54.1 to 44.5 kJ mol−1, respectively, when the CO2 pressure increased from 0.1 to 8 MPa. It is indicated that the curing reaction of epoxy resin occurs more easily under higher-pressure CO2 due to the promoted molecular movement, especially in the supercritical state. The non-isothermal curing kinetics were investigated by nth-order model and two-parameter autocatalytic model (Šesták–Berggren model), and the latter one analyzed by Málek method exhibited good agreement with the experimental data except the initial and final stages of the curing reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the high electrical insulativity, chemical resistance, adhesion and high tensile strength, epoxy resin is widely applied as electronics, coatings, adhesives and composites, which is regarded as one of the most important thermosetting polymers [1, 2]. The properties of epoxy resins are significantly affected by the curing reaction which is determined by curing temperature, time and reaction medium [3]; thus, the reaction kinetics should be studied. However, the multi-process during the curing reaction involving ring-opening of epoxy resin, chain growth of amines and chain branching leads to the complicated reaction kinetics.
On the other hand, carbon dioxide (CO2) is used as a desirable solvent in polymerization reaction and polymer process [4], which can reduce the viscosities and the mass transfer resistance of the polymer reagents, leading a higher conversion. Besides, the diffusion rate of polymers is enhanced by the swelling of CO2, leading to the increase in reaction rate [5]. In some recent studies [6,7,8], epoxy resin samples were more and more post-cured and foamed under high-pressure CO2 instead of traditional solvents or blowing agents. The polymerization in the presence of CO2 has been widely studied for the production of polystyrene [9], polycarbonate [10], polyamide [11] and polymethyl methacrylate [12]. However, the curing reaction of epoxy resin under CO2 has not been studied yet.
In general, the non-isothermal process is conducted to research the curing kinetics for many polymers [13,14,15]. Non-isothermal process is performed in a wide temperature range, and the completed curing reaction is ensured. As reported, differential scanning calorimetry (DSC) [16,17,18] and Fourier transform infrared spectroscopy (FTIR) [19,20,21] are frequently used for the analysis of the epoxy resin non-isothermal curing kinetics. FTIR analysis is a direct method which reflects the assessment of reactive groups as a function of time, while DSC as an indirect method gives the thermal property directly from which the relationship between reactants’ conversion and reaction time can be obtained [22].
Isoconversional methods are usually used to evaluate the activation energy, E a, ignoring the reaction models since the reaction rate at constant extent of conversion is the only function of temperature [23]. A number of equations give the relationship between temperature and conversion following the isoconversional principle, such as Friedman [24], Ozawa–Flynn–Wall [25, 26], Kissinger–Akahira–Sunose (KAS) [27] and Starink [28] equations. Roudsari [29] et al. selected three different isoconversional equations to obtain the activation energy and found that the results from KAS and Starink methods were more appropriate than that from Friedman method for the DGEBA-based epoxy/sebacic acid systems. Moreover, Vyazovkin [30,31,32] developed a numerical integration method to improve the accuracy, and many researches adopted this method [33,34,35]. Moreover, many reports proposed that the curing kinetics of epoxy resin were studied by several analytical method and kinetic models. Sun and Wang [36] discussed the nth-order and two-parameter autocatalytic model for the mixed epoxy systems with different ratios and indicated that the latter model well described the reaction process. Fan et al. [33] studied the non-isothermal and isothermal curing kinetics of DGEBA epoxy system with DSC and used graphics analytical method to determine the kinetics parameters. They found Šesták–Berggren model and Kamal model suitable for the non-isothermal and isothermal reaction, respectively. These researches mentioned above were all conducted under atmospheric N2; however, there is no report published on the epoxy resin curing behavior under high-pressure atmosphere.
Therefore, it is worth to conduct a systemic work on the curing kinetics of the epoxy resin under high-pressure CO2 for a better understanding of the complicated curing process and the effect of CO2. In this work, the non-isothermal curing kinetics of epoxy resin consisting of DGEBA and aromatic diamine were built by DSC method under compressed CO2. The curing activation energies were explored by both KAS plots method and Vyazovkin isoconversional method to investigate the effect of CO2 pressure on curing reaction behavior. Furthermore, the kinetics parameters were calculated from nth-order and two-parameter autocatalytic models.
Experimental
Materials
The diglycidyl ether of bisphenol A (DGEBA), E51, with epoxy equivalent weight of 196 g/equiv, was purchased from Hangzhou Wuhuigang Adhesive, China. The m-xylylenediamine (MXDA, 99% pure), used as a curing agent, was supplied by Aladdin Industrial Corporation, China. CO2 (99.995% pure) and N2 (99.99% pure) were supplied by Shanghai Chenggong gas industry, China.
Samples preparation
The epoxy resin and curing agent were mixed in a beaker at a theoretically calculated mixing mass ratio of 100:17 and stirred mechanically at room temperature for about 5 min. Before tests, the homogeneous solution was stored below 273 K to avoid curing reaction.
Characterization
The reaction processes were characterized by in situ FTIR (BRUKER EQUINOX-55, Germany) equipped with a high-pressure component (HARRICK, USA). The solution samples were coated on the SeZn windows with a thickness of 100 μm and then swept with test gas for three times to replace the air. In this paper, all the samples were kept under the specific pressure at 298 K for 60 min to dissolve gas fully before every curing process. The sample was heated from 298 to 453 K at the heating rate of 5 K min−1, and the spectrum was acquired per minute during curing. The FTIR spectra were recorded per minute at the wave number of 4000–400 cm−1. One spectrum was scanned per 32 s at a resolution of 4.0 cm−1.
The non-isothermal curing process was performed by high-pressure DSC (NETZSCH DSC 204 HP, Germany) under compressed CO2 (0.1–8 MPa). Before curing measurements, the curves were calibrated with In, Bi, Sn, Pb and Zn at compressed CO2. The samples, weighed 5–10 mg, were placed in aluminum pans and sealed. After swept with test gas and dissolved, the samples were heated from 298 to 473 K at varied heating rate of 5, 10, 15 and 20 K min−1.
Results and discussion
Comparison of curing reaction under N2 and CO2
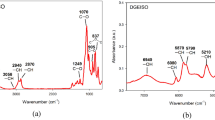
The curing reaction was conducted under different conditions in in situ FTIR to compare the reaction processes under atmospheric N2 and high-pressure CO2, and the spectra were obtained every minute during heating from 298 to 453 K as shown in Fig. 1. In the spectrum of un-cured sample, the band at 1509 cm−1 is the stretching vibration peak of C=C in benzene skeleton. The bands at 1297, 1248 and 1184 cm−1 are characteristic peaks of ether group, the band at 915 cm−1 is the characteristic peak of oxirane ring, and the band at 833 cm−1 is the p-phenylene group absorption peak. Owing to the ring-opening reaction, the peak area of oxirane ring (915 cm−1) decreases during the curing process and is not presented in the spectrum of completely cured sample. The spectra of reaction processes obtained under N2 and CO2 are almost the same. So the presence of CO2 has no effect on reaction components in epoxy resin curing process.
Non-isothermal curing kinetic equations
The reaction rate can be parameterized with the so-called reaction kinetics in accordance with the temperature, T, and the conversion degree, α [23, 37],
According to Arrhenius equation, k(T) is described as follows:
where E a is the apparent activation energy, A is the pre-exponential factor and R is the gas constant, 8.3145 J/(mol K). Reaction model f(α) of epoxy curing is usually hypothesized to follow nth-order reaction model (Eq. 3) and two-parameter autocatalytic reaction model (Šesták–Berggren model, Eq. 4) [36, 38],
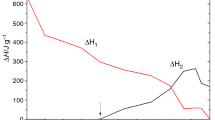
where α is the fractional conversion at a certain time and calculated as follows:
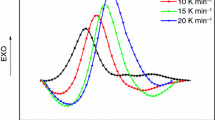
the fractional enthalpy, \(\Delta H\left( t \right)\), and the total enthalpy, \(\Delta H_{\text{total}}\), of epoxy curing reaction can be obtained from DSC exothermal curves. The DSC curves of epoxy resin curing at different heating rates of 5, 10, 15 and 20 K min−1 under CO2 with the pressure of 0.1 MPa are shown in Fig. 2, and the onset temperature (T o), peak temperature (T p), end temperature (T e) and reaction heating enthalpy (ΔH) of the exothermal curves obtained are listed in Table 1. In Fig. 2 and Table 1, it is showed that the exothermal peaks shift toward the higher temperatures as the heating rates increase, due to the thermal lag effect. Furthermore, the enthalpy of reaction increases with the higher heating rate, which is consistent with the results in Sun and Wang [36] and Sun et al. [39] work.
The conversions as a function of temperature at the heating rate of 5 K min−1 under different CO2 pressures are shown in Fig. 3. It indicates that there is little difference between the reaction processes under 0.1 and 2 MPa. And the increase in conversion slightly accelerates with increasing pressure from 4 to 8 MPa. However, the reaction temperature and apparent activation energy are changing during the non-isothermal curing reaction, which leads to the complicated influence on the conversion. In this study, the apparent activation energy is only estimated, while the effect of CO2 pressure on reaction rate will be investigated through isothermal process in the further study.
Activation energy of non-isothermal curing kinetics
Kissinger–Akahira–Sunose (KAS) method
Isoconversional methods are the most common methods for analyzing the activation energy, in which reaction temperature is the only factor determining the reaction rate at constant conversion degree [23]. E a is calculated by integral isoconversional KAS method [27, 28], which is more popular and accurate for epoxy resin system [29, 39, 40].
where β is the heating rate and T α is the temperature of the sample with a certain conversion degree. Figure 4 shows the KAS plots of \(\ln \left( {\beta /T^{2} } \right)\) versus \(1000/T\) at the conversion degree of 5, 10, 15… 90, 95 and 98% under 0.1 MPa CO2. The E a calculated from the slopes of these lines are present in Fig. 5, and the average value of E a decreases from 53.5 to 46.8 kJ mol−1 with CO2 pressure increasing from 0.1 to 8 MPa. It can be seen that three stages (stages I, II, and III) are expressed in the curves of E a value. With conversion increases from 5 to 30% (stage I), the activation energies enlarge due to the rapid increase in polymer chain and molecular weight. The high viscosity resists the mixing and reaction of reactants. At the conversion range from 30 to 80% (stage II), both positive and negative effects of elevated temperature and chain growth on the energetic barriers lead to a stable value of activation energy. In the stage III of reaction (α > 80%), it is difficult for reaction as a result of high cross-link density and lack of reactive groups. Furthermore, it indicates that the curing reaction occurs more easily over lower energy barriers under higher pressure. Dissolved CO2 in epoxy resin system proves to accelerate the curing reaction occurring. When epoxy resin system reacts at 2–6 MPa (gas CO2 stage), the E a is nearly the same. In this case, CO2 gas has no solvent effect on the epoxy system, and a small amount of dissolved CO2 can improve the curing reaction slightly. With increasing CO2 pressure, the pressure affects the fluid condition in this system. In supercritical state at the pressure of 8 MPa, the E a decreases against the CO2 pressure significantly. In addition, molecules movement and cross-linking are intensified with the higher pressure in supercritical CO2, resulting in declining energy barrier.
Vyazovkin method
Vyazovkin method is calculated by a direct numerical integration. The value of E a is the minimum of the following function at various heating rates,
where I is the temperature integral, evaluated numerically,
Figure 6 shows the dependence of E α on α acquired by the minimization repeated for α values between 0.01 and 0.99 with a step size of Δα = 0.01. Consistent with the result obtained by KAS method, there are also three stages in the variation of E α. The curing reaction initiates at low activation energy; then, the E α increases significantly in the stage I. After a stabilization stage (stage II), E α enlarges with the cross-link reaction in the stage III. And the average values of E α are present in Fig. 6b, which indicates that the activation energy decreases from 54.1 to 44.5 kJ mol−1 with CO2 pressure increasing from 0.1 to 8 MPa. Furthermore, the values of activation energy calculated by Vyazovkin method approach to the ones by KAS method.
Model-fitting kinetics analysis
The nth-order model (Eq. 3) and two-parameter autocatalytic reaction model (Eq. 4) are usually applied to investigate the non-isothermal curing process of epoxy system. However, the former (calculated in supporting information) is not suitable for the curing reaction of the epoxy system in this study, since the complicated process involving gelation, glass transition, diffusion control and side reaction is not idealized [36, 41].
Šesták–Berggren autocatalytic model [38] is an empirical model depending on different reaction orders, m and n. To analyze the autocatalytic model, Málek method [23, 42] is usually used to determine m and n by two special characteristic functions, y(α) and z(α) [36, 43],
where \(x = E_{\text{a}} /RT\) and π(x) is the integral for temperature, usually calculated by expression of Senum–Yang [44],
The value of E a determined by Vyazovkin method is substituted into Eqs. 9 and 10; then, the function curves of y(α) and z(α) are obtained and plotted in Fig. 7. \(\alpha_{\text{M}}\), \(\alpha_{\text{P}}^{\infty }\) and \(\alpha_{\text{P}}\) are conversion degrees of maximum y(α), z(α) and dα/dt, respectively. The mean values (in Table 2) calculated under different heating rates are used to analyze the kinetics equations, which are subject to satisfaction of the following conditions: \(0 < \alpha_{\text{M}} < \alpha_{\text{P}}^{\infty }\) and \(\alpha_{\text{P}}^{\infty } \ne 0.632\). According to the criterion of Málek method, the curing process in this work proves to be consistent with the autocatalytic reaction model (Eq. 4).
The Šesták–Berggren autocatalytic model can be expressed as follows by combining Eqs. 1, 2 and 4,
and Eq. 12 is usually transformed as follows when \(p = \alpha_{\text{M}} /\left( {1 - \alpha_{\text{M}} } \right) = m/n\).
Figure 8 shows the linear relationship between \(\ln \left[ {\left( {{{{\text{d}}\alpha } \mathord{\left/ {\vphantom {{{\text{d}}\alpha } {{\text{d}}t}}} \right. \kern-0pt} {{\text{d}}t}}} \right)\exp \left( {{{E_{\text{a}} } \mathord{\left/ {\vphantom {{E_{\text{a}} } {RT}}} \right. \kern-0pt} {RT}}} \right)} \right]\) and \(\ln \left[ {\alpha^{\text{p}} \left( {1 - \alpha } \right)} \right]\) at \(0.2 \le \alpha \le 0.95\). Table 3 lists the values of m, n and A obtained from the slopes and intercepts of the fitting lines at different heating rates under 0.1 MPa CO2.
Curves of \(\ln \left[ {\left( {{{{\text{d}}\alpha } \mathord{\left/ {\vphantom {{{\text{d}}\alpha } {{\text{d}}t}}} \right. \kern-0pt} {{\text{d}}t}}} \right)\exp \left( {{{E_{\text{a}} } \mathord{\left/ {\vphantom {{E_{\text{a}} } {RT}}} \right. \kern-0pt} {RT}}} \right)} \right]\) versus \(\ln \left[ {\alpha^{\text{P}} \left( {1 - \alpha } \right)} \right]\) with different heating rates under 0.1 MPa CO2
Above all, the parameters of non-isothermal curing kinetics under different CO2 pressures are listed in Table 4, and the simulated model (lines) is consistent with the experimental data (dots) as shown in Fig. 9. These results indicate that Šesták–Berggren autocatalytic model can properly describe non-isothermal curing of the chosen epoxy resin system under different CO2 pressures in this work. Nevertheless, deviations appear in the initial and final stages due to the rapid increase in molecular weight and gas diffusion [36], which is in accordance with the variation of activation energy.
Conclusions
The non-isothermal curing behavior of epoxy system consisting of DGEBA and MXDA under high-pressure CO2 was studied by DSC. The CO2 dissolved in the epoxy system exhibits no effect on the reaction components in epoxy resin curing process, but enhances the molecular movement and the collision among reactant components that lead to curing reaction occurs more easily. So the activation energies of non-isothermal curing reaction decrease with increasing CO2 pressure. Moreover, the activation energies obtained by both KAS method and Vyazovkin method increase in the stage I, then stay stable in the stage II, and increase continuously in the stage III with increasing conversion. The nth-order model is not applicable for the non-isothermal curing reaction, while the Šesták–Berggren autocatalytic model well describes the process under CO2 from 0.1 to 8 MPa.
References
Saad GR, Abd Elhamid EE, Elmenyawy SA. Dynamic cure kinetics and thermal degradation of brominated epoxy resin–organoclay based nanocomposites. Thermochim Acta. 2011;524(1–2):186–93.
Wu CC, Hsu SLC. Thermo-curable epoxy systems for nanoimprint lithography. J Micromech Microeng. 2010;20(1):015006.
Li Q, Li X, Meng Y. Curing of DGEBA epoxy using a phenol-terminated hyperbranched curing agent: cure kinetics, gelation, and the TTT cure diagram. Thermochim Acta. 2012;549:69–80.
Kendall JL, Canelas DA, Young JL, DeSimone JM. Polymerizations in supercritical carbon dioxide. Chem Rev. 1999;99(2):543–64.
Nalawade SP, Picchioni F, Janssen LPBM. Supercritical carbon dioxide as a green solvent for processing polymer melts: processing aspects and applications. Prog Polym Sci. 2006;31(1):19–43.
Ito A, Semba T, Taki K, Ohshima M. Effect of the molecular weight between crosslinks of thermally cured epoxy resins on the CO2-bubble nucleation in a batch physical foaming process. J Appl Polym Sci. 2014;131(12):222–4.
Lyu J, Liu T, Xi Z, Zhao L. Effect of pre-curing process on epoxy resin foaming using carbon dioxide as blowing agent. J Cell Plast. 2016;53(2):181–97.
Ren Q, Xu H, Yu Q, Zhu S. Development of epoxy foaming with CO2 as latent blowing agent and principle in selection of amine curing agent. Ind Eng Chem Res. 2015;54(44):11056–64.
Canelas DA, DeSimone JM. Dispersion polymerizations of styrene in carbon dioxide stabilized with poly(styrene-b-dimethylsiloxane). Macromolecules. 1997;30(19):5673–82.
Gross SM, Flowers D, Roberts G, Kiserow DJ, DeSimone JM. Solid-state polymerization of polycarbonates using supercritical CO2. Macromolecules. 1999;32(9):3167–9.
Givens RD, Jikei M, DeSimone JM. Synthesis of polyamides and polycarbonates using supercritical CO2. Polym Prepr. 1997;38:468–9.
Cooper AI, Hems WP, Holmes AB. Synthesis of highly cross-linked polymers in supercritical carbon dioxide by heterogeneous polymerization. Macromolecules. 1999;32(7):2156–66.
Ng H, Manas-zloczower I. A nonisothermal differential scanning calorimetry study of the curing kinetics of an unsaturated polyester system. Polym Eng Sci. 1989;29(16):1097–102.
Bai Y, Yang P, Zhang S, Li Y, Gu Y. Curing kinetics of phenolphthalein–aniline-based benzoxazine investigated by non-isothermal differential scanning calorimetry. J Therm Anal Calorim. 2015;120(3):1755–64.
Parthun MG, Johari GP. Relaxations in thermosets. 24. Theory and experiments on nonisothermal curing of thermoset polymers. Macromolecules. 1992;25(12):3149–55.
Xu X, Zhou Q, Song N, Ni Q, Ni L. Kinetic analysis of isothermal curing of unsaturated polyester resin catalyzed with tert-butyl peroxybenzoate and cobalt octoate by differential scanning calorimetry. J Therm Anal Calorim. 2017;. doi:10.1007/s10973-017-6246-z.
Morancho JM, Fernández-Francos X, Acebo C, Ramis X, Salla JM, Serra À. Thermal curing of an epoxy-anhydride system modified with hyperbranched poly(ethylene imine)s with different terminal groups. J Therm Anal Calorim. 2017;127(1):645–54.
Lee JY, Choi HK, Shim MJ, Kim SW. Kinetic studies of an epoxy cure reaction by isothermal DSC analysis. Thermochim Acta. 2000;343(1–2):111–7.
Mustata F, Tudorachi N. Thermal behavior of epoxy resin cured with aromatic dicarboxylic acids. J Therm Anal Calorim. 2016;125(1):97–110.
Li F, Zhou S, You B, Wu L. Kinetic study on the UV-induced photopolymerization of epoxy acrylate/TiO2 nanocomposites by FTIR spectroscopy. J Appl Polym Sci. 2006;99(6):3281–7.
Tadros R, Timm DC. Fourier-transform infrared analysis of a linear, anhydride-cured epoxy. Macromolecules. 1995;28(22):7441–6.
Achilias DS, Karabela MM, Varkopoulou EA, Sideridou ID. Cure kinetics study of two epoxy systems with fourier tranform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC). J Macromol Sci Pure. 2012;49(8):630–8.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1–2):1–19.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci Part C Polym Sympos. 1964;6(1):183–95.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand Phys Chem. 1966;70A(6):487–523.
Akahira T, Sunose T. Method of determining activation deterioration constant of electrical insulating materials. Res Rep Chiba Inst Technol (Sci Technol). 1971;16:22–31.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404(1–2):163–76.
Roudsari GM, Mohanty AK, Misra M. Study of the curing kinetics of epoxy resins with biobased hardener and epoxidized soybean oil. ACS Sustain Chem Eng. 2014;2(9):2111–6.
Vyazovkin S. Advanced isoconversional method. J Therm Anal. 1997;49(3):1493–9.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22(2):178–83.
Vyazovkin S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J Comput Chem. 1997;18(18):393–402.
Fan M, Li X, Zhang J, Cheng J. Curing kinetics and shape-memory behavior of an intrinsically toughened epoxy resin system. J Therm Anal Calorim. 2015;119(1):537–46.
Alzina C, Sbirrazzuoli N, Mija A. Hybrid nanocomposites: advanced nonlinear method for calculating key kinetic parameters of complex cure kinetics. J Phys Chem B. 2010;114(39):12480–7.
Sbirrazzuoli N. Is the friedman method applicable to transformations with temperature dependent reaction heat? Macromol Chem Phys. 2007;208(14):1592–7.
Sun JS, Wang JG. Study on the optimum ratio and non-isothermal curing kinetics of EP2008/EP2008-S system. J Therm Anal Calorim. 2014;118(1):571–8.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, et al. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3(1):1–12.
Sun H, Liu Y, Wang Y, Tan H. Curing behavior of epoxy resins in two-stage curing process by non-isothermal differential scanning calorimetry kinetics method. J Appl Polym Sci. 2014;131(17):8761–8.
Xiong X, Ren R, Liu S, Lu S, Chen P. The curing kinetics and thermal properties of epoxy resins cured by aromatic diamine with hetero-cyclic side chain structure. Thermochim Acta. 2014;595:22–7.
Wan J, Bu ZY, Xu CJ, Li BG, Fan H. Learning about novel amine-adduct curing agents for epoxy resins: butyl-glycidylether-modified poly(propyleneimine) dendrimers. Thermochim Acta. 2011;519(1–2):72–82.
Hardis R, Jessop JLP, Peters FE, Kessler MR. Cure kinetics characterization and monitoring of an epoxy resin using DSC, Raman spectroscopy, and DEA. Compos Part A Appl Sci. 2013;49:100–8.
Málek J. The kinetic analysis of non-isothermal data. Thermochim Acta. 1992;200:257–69.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal. 1977;11(3):445–7.
Acknowledgements
This work is financial supported by the National Key Research and Development Program of China (2016YFB0302201) and the Plan for Shanghai excellent technical leaders (14xd425500).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lyu, J., Hu, D., Liu, T. et al. Non-isothermal kinetics of epoxy resin curing reaction under compressed CO2 . J Therm Anal Calorim 131, 1499–1507 (2018). https://doi.org/10.1007/s10973-017-6574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6574-z