Abstract
The crystallization behavior of partially melted poly(ether ether ketone) (PEEK) was investigated by differential scanning calorimetry. The influence of pre-crystallization conditions was studied in details. After partial melting, the maximum peak shifts to higher temperature by 8.4–11.8 °C during subsequent cooling, indicating that self-nucleation remarkably enhanced the overall crystallization rate of PEEK. An interesting finding is that the self-nucleation temperature domain of PEEK is way outside the melting curve, and its magnitude is greatly large (28–35 °C). Moreover, samples that were pre-crystallized nonisothermally or from the glassy state exhibit a larger self-nucleation effect, and a larger self-nucleation temperature domain. The isothermal crystallization data were analyzed by the Avrami model. The Avrami exponent is found to be remarkably different for melts with and without self-nucleation effects, indicating that self-nucleation changes the crystallization mechanism of PEEK.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has been well established that primary nucleation is the crucial step to initiate the crystallization of polymers [1, 2]. There are two types of primary nucleation: homogeneous and heterogeneous nucleation. Homogeneous nucleation can be defined by spontaneous aggregation of polymer molecules to form a three-dimensional nucleus, in which large supercooling is required. While in heterogeneous nucleation, nucleation sites preexist in a sample, which can lower the surface free energy barrier toward nucleation, and thus initiate crystallization at higher temperature during cooling. This can usually be achieved through the addition of nucleating agents. Alternatively, self-nucleation has been demonstrated as an effective approach to overcome the problem of nucleation and to avoid large supercooling [1–13]. Self-nucleation typically refers to clusters of molecules that retain their crystallographic arrangement as a result of partial melting, and on subsequent cooling, these aggregates of clusters of molecules can act as predetermined athermal nucleation sites which can greatly enhance the overall crystallization rate of polymers [4–6]. For example, after partial melting, the maximum crystallization peak shifts to higher temperature by 30 °C during subsequent cooling for poly(lactic acid) (PLA), which is a polyester with intrinsically slow crystallization rate [7].
Self-nucleation effect has been studied as a function of experimental variables such as heating rate, melting temperature and time [4–14]. Among the variables, melting temperature plays a critical role on the self-nucleation effect. According to Fillon et al. [5], self-nucleation temperature range (denoted T s) or domain II corresponds to the temperatures that are high enough to melt almost all crystals in the sample, but low enough to leave small crystal self-nuclei that can nucleate the polymer upon subsequent cooling from T s. It is characterized by an exponential increase in nucleation density and a corresponding shift of the peak crystallization temperature to higher values. In contrast, domain III occurs at lower temperatures where partial melting and annealing take place simultaneously, while domain I is defined as the temperature range where complete melting is achieved [5]. It is noteworthy that the T s range is strongly dependent on the chemical structures of polymers [4, 5, 13]. For example, for isotactic polypropylene (iPP) the T s range is rather narrow (4 °C), and the domain is located between the maximum and its high-temperature foot of the melting peak [4, 5]. While for polyvinylidene fluoride (PVDF), the T s range can be very large (15 °C) and its upper limit is far beyond the high-temperature foot of the melting curve [13].
Besides, the pre-crystallization conditions also play an important role on the self-nucleation effect of polymers [7, 12, 15, 16]. Maus et al. [12] investigated the self-nucleation behavior of syndiotactic polypropylene (sPP) pre-crystallized isothermally from the melt at various crystallization temperatures. It was found that the samples pre-crystallized at lower temperature exhibited a larger self-nucleation effect. For PLA, the self-nucleation behaviors of as received samples [15], single crystals [16] and the ones crystallized from the melt nonisothermally [7] were also reported in the literature.
Poly(ether ether ketone) (PEEK) is a high-performance engineering thermoplastic polymer, widely used in aerospace, automotive, electronics and chemical industries [17–20]. Nevertheless, a high degree of crystallinity is required to ensure optimal mechanical performances [21–23]. The understanding of the crystallization behavior allows a better control and optimization of the crystallinity and crystalline structure of polymers. In this context, the isothermal and nonisothermal behaviors of PEEK have been investigated extensively [24–32]. However, little work has been carried out to date on the influence of self-nucleation on the crystallization behavior of PEEK [33]. Tan et al. [33] investigated the crystallization kinetics of PEEK from its metastable melt, obtained from partial melting between the double endotherm. After partial melting, the type of lamellar crystal exhibiting the higher thermodynamic stability remained and the metastable melt recrystallized immediately just above the lower melting temperature and formed slightly thicker lamellae than the original ones [33].
In this paper, the influence of pre-crystallization conditions on the self-nucleation behavior of poly(ether ether ketone) (PEEK) was investigated by differential scanning calorimetry (DSC). Since PEEK can be crystallized either from the melt or the glassy state [28, 30, 32], it provides an adequate model to investigate the influence of pre-crystallization conditions on the self-nucleation behavior of polymers. In general, the spherulites in melt-crystallized polymers are much larger than those in cold-crystallized counterparts [34, 35]. Moreover, a single polymer spherulite usually exhibits radial inhomogeneity on a micrometer scale [36]. In this context, the self-nucleation behavior of melt-crystallized and cold-crystallized PEEK samples may be different.
Experimental
Materials
The PEEK was purchased from Sigma–Aldrich. Its number- and weight-average molecular weights were 1.03 × 104 and 2.08 × 104, respectively.
DSC measurements
A DSC 2920 (TA Instruments) was used to study the crystallization behavior of PEEK. Calibration for the temperature and energy scales was carried out using a pure indium standard. Sample weight was about 5 mg. Each sample was used only once, and all the runs were carried out in a nitrogen atmosphere. Pre-crystallized PEEK samples were prepared by nonisothermal or isothermal crystallization from the melt or the glassy state, respectively. In details, the samples were first heated to 400 °C at 20 K min−1 and held for 5 min to erase previous thermal history; then, they were (a). cooled to 240 °C at 10 K min−1; or (b). cooled rapidly to 320 °C and held for 30 min; or (c). quenched into ice water to obtain amorphous state and then reheated at 10 K min−1; or (d). quenched into ice water and then cold-crystallized at 159 °C for 30 min, to obtain the pre-crystallized samples. To observe the melting behavior of the pre-crystallized samples, the DSC scans were recorded further during subsequent heating at 10 K min−1.
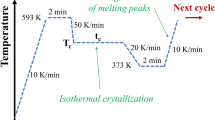
As relevant for determining the partial melting temperature (T s) range of the pre-crystallized PEEK, Fig. 1 illustrates a representative thermal program employed for self-nucleation experiment of the sample pre-crystallized nonisothermally from the melt. The sample was first heated to 400 °C at 20 K min−1 (step 1) and held for 5 min (step 2); then, it was cooled to 240 °C at 10 K min−1 to obtain the pre-crystallized sample (step 3). The pre-treated sample was heated at 10 K min−1 to T s (step 4) and held for 5 min to create self-nucleated sites (step 5). Then, it was cooled to 240 °C at 10 K min−1, and the crystallization peak temperature of self-nucleated PEEK was determined (step 6). Finally, the sample was reheated to 400 °C at 10 K min−1 (step 7). This program is adapted to other pre-crystallized conditions (step 3 is modified, also the levels of steps 5 and 6).
The isothermal crystallization behavior of self-nucleated PEEK was also investigated. Following the steps 1–3 (see Fig. 1), the nonisothermally pre-crystallized sample from the melt was obtained. Then, it was heated at 10 K min−1 to 350 °C (step 4) and held for 5 min to create self-nucleated sites (step 5). Finally, the sample was cooled rapidly to the selected crystallization temperature (T c), and the DSC traces were recorded during the isothermal process. The T c was selected based on a rule that it could give a reliable crystallization exotherm while the completion of crystallization did not take too long. As a reference, the isothermal crystallization behavior of PEEK without pre-existing nuclei was also investigated. The samples were heated to 400 °C at 20 K min−1 and held for 5 min to erase previous thermal history. Then, they were cooled rapidly to the selected T c, and the DSC traces were recorded during the isothermal process.
Results and discussion
Melting temperature as a function of pre-crystallization conditions
As a start, we examine first the melting behavior of PEEK samples prepared under various conditions. As shown in Fig. 2, PEEK pre-crystallized nonisothermally or isothermally from the melt presents a melting peak at 342.0 or 346.8 °C, respectively. For PEEK pre-crystallized nonisothermally or isothermally from the glass, its melting peak appears at 341.9 or 342.3 °C, respectively. Obviously, the melting curve is broader for the samples pre-crystallized from the glassy state.
DSC heating scans (at 10 K min−1) of PEEK samples pre-crystallized (a) nonisothermally and (b) isothermally from the melt, and (c) nonisothermally and (d) isothermally from the glass, respectively. The self-nucleation range of each sample is also indicated. Note that the curves are only shifted for clarity reason
Self-nucleation domains as a function of pre-crystallization conditions
The above comparison of melting peak temperatures deals with only one aspect of the melting behavior of the polymers. Comparison of the self-nucleation domains makes it possible to cover the broader spectrum of thermal stabilities of crystals present in any pre-crystallized PEEK sample. Two criteria have been used to define this nucleation range: the magnitude of the range and its position relative to the melting peak [13]. For the sample pre-crystallized from the melt nonisothermally, the DSC curves of self-nucleated PEEK are presented in Fig. 3. Figure 3a shows the cooling runs at 10 K min−1 after thermal conditioning at the indicated melting temperatures for 5 min, and Fig. 3b shows the subsequent heating runs at 10 K min−1. After being melted at temperatures between 400 and 378 °C, all the DSC cooling profiles present one exothermic peak at around 296.5 °C, while the subsequent heating profiles reveal one melting peak also. This indicates that 378 °C is the lowest temperature at which complete melting occurs. Thermal conditioning at 377 °C was able to self-nucleate PEEK and, therefore, its crystallization temperature shifts to 298.9 °C. With further decrease in the melting temperature, its crystallization temperature shifts to higher levels and reaches 307.5 °C for a melting temperature of 348 °C. At a melting temperature of 347 °C, a weak crystallization peak and double melting peaks are present on cooling and subsequent heating, respectively. With further decrease in the melting temperature, its crystallization peak gets weaker on cooling. During the subsequent heating process, the lower endotherm gets weaker while the higher one gets stronger. This can be explained as follows: the sample was not sufficiently melted at or below 348 °C so that self-nucleation and annealing of un-melted crystals took place simultaneously. Similar multiple endothermic peaks after self-nucleation of PP [5, 9] and PLA [7] have been reported. These results clearly show that partial melting temperature 348 °C corresponds to the highest achievable crystallization peak temperature of PEEK during cooling. From above results, it can be concluded that, for PEEK pre-crystallized nonisothermally from the melt, the corresponding magnitude of the T s range (348–378 °C) can be obtained to be 30 °C and the maximum peak shifts to higher temperature by 11.0 °C.
DSC curves of PEEK pre-crystallized nonisothermally from the melt: a during cooling runs at 10 K min−1 after thermal conditioning at the indicated melting temperatures for 5 min, and b the subsequent heating runs at 10 K min−1. Note that the nonisothermal pre-crystallization from the melt corresponds to a cooling at 10 K min−1 down to 240 °C, the cooling run at 10 K min−1 after partial melting corresponds to step 6 (see Fig. 1), the partial melting is step 5 and the subsequent heating at 10 K min−1 is step 7. The curves are only shifted for clarity reason
For the samples pre-crystallized from other thermal conditions, the DSC curves of self-nucleated PEEK are presented in Figs. 4–6. For PEEK pre-crystallized isothermally from the melt, the corresponding magnitude of the T s range (350–378 °C) can be obtained to be 28 °C and the maximum peak shifts to higher temperature by 8.4 °C during subsequent cooling (Fig. 4). While for PEEK pre-crystallized nonisothermally from the glass, the magnitude of the T s range (349–384 °C) is 35 °C and the maximum peak shifts to higher temperature by 11.8 °C during subsequent cooling (Fig. 5). And while for PEEK pre-crystallized isothermally from the glass, the magnitude of the T s range (350–382 °C) is 32 °C and the maximum peak shifts to higher temperature by 10.7 °C during subsequent cooling (Fig. 6). The T s ranges of PEEK pre-crystallized at various conditions are also indicated in Fig. 2.
DSC curves of PEEK pre-crystallized isothermally from the melt: a during cooling runs at 10 K min−1 after thermal conditioning at the indicated melting temperatures for 5 min, and b the subsequent heating runs at 10 K min−1. Note that the isothermal pre-crystallization from the melt corresponds to a rapid cooling down to 320 °C and holding at the temperature for 30 min, the cooling run at 10 K min−1 after partial melting corresponds to step 6 (see Fig. 1), the partial melting is step 5 and the subsequent heating at 10 K min−1 is step 7. The curves are only shifted for clarity reason
DSC curves of PEEK pre-crystallized nonisothermally from the glass: a during cooling runs at 10 K min−1 after thermal conditioning at the indicated melting temperatures for 5 min, and b the subsequent heating runs at 10 K min−1. Note that the nonisothermal pre-crystallization from the glass corresponds to quenching into ice water from the melt to obtain amorphous state and then reheating at 10 K min−1 to finish cold crystallization, the cooling run at 10 K min−1 after partial melting corresponds to step 6 (see Fig. 1), the partial melting is step 5 and the subsequent heating at 10 K min−1 is step 7. The curves are only shifted for clarity reason
DSC curves of PEEK pre-crystallized isothermally from the glass: a during cooling runs at 10 K min−1 after thermal conditioning at the indicated melting temperatures for 5 min, and b the subsequent heating runs at 10 K min−1. Note that the isothermal pre-crystallization from the glass corresponds to quenching into ice water from the melt to obtain amorphous state and then holding at 159 °C for 30 min to finish cold crystallization, the cooling run at 10 K min−1 after partial melting corresponds to step 6 (see Fig. 1), the partial melting is step 5 and the subsequent heating at 10 K min−1 is step 7. Note that the curves are only shifted for clarity reason
From above results, it can be concluded that, after partial melting, the maximum crystallization peak shifts to higher temperatures by 8.4–11.8 °C, indicating that self-nucleation significantly enhances the overall crystallization rate of PEEK. Moreover, the self-nucleation effect is more significant for the samples pre-crystallized nonisothermally or from the glassy state. On the other hand, the magnitude of the T s range varies from 28 to 35 °C, depending on the pre-crystallization conditions. The range is larger for the samples pre-crystallized nonisothermally or from the glassy state. It is noteworthy that the magnitude of the T s range is rather narrow for iPP (4 °C) and even more so for polyethylene (1.5 °C), and the domain is usually located between the high-temperature foot of the melting peak and its maximum [4, 5]. For PVDF, the T s range can be larger (15 °C) and its upper limit is beyond the high-temperature foot of the melting curve [13]. Interestingly, the self-nucleation temperature domain of PEEK is way outside the melting curve, and its magnitude is greatly large (28–35 °C).
Isothermal crystallization kinetics of self-nucleated PEEK
The crystallization kinetics of self-nucleated PEEK was investigated further. The samples melt-crystallized nonisothermally were melted partially at 350 °C for 5 min to create the self-nucleation site. Figure 7a, b presents the isothermal crystallization curves of PEEK at various crystallization temperatures (T c) after completely melted at 400 °C for 5 min and partially melted at 350 °C for 5 min, respectively. For both completely and partially melted PEEK samples, the subsequent isothermal crystallization exotherm gets broader with increasing T c, although the investigated T c range was much lower for the completely melted PEEK than for the partially melted one.
The relative degree of crystallinity X(t), as a function of crystallization time t, can be calculated from the integrated area of the DSC curve from t = 0 to t divided by the integrated area of the whole heat flow curve. Figure 8 shows the plots of X(t) as a function of t for completely and partially melted PEEK samples crystallized at indicated crystallization temperatures. An important parameter which can be taken directly from Fig. 8 is the halftime of crystallization t 0.5, which is the change in time from the onset of crystallization to the time at which X(t) is 50%. The values are summarized in Table 1. The t 0.5 values increase with increasing T c for both PEEK samples, indicating that the overall isothermal crystallization rate decreases with increasing T c. Such results are reasonable since it is difficult for the samples to nucleate at high T c, thereby resulting in the reduction in the overall crystallization rate. At the same T c, the t 0.5 value of PEEK with self-nucleation is much lower than that of the one without, indicating that self-nucleation accelerates the crystallization of PEEK.
The well-known Avrami equation [37, 38] was used to describe the overall isothermal crystallization kinetics, which is shown as follows:
where n is the Avrami index, and k is the overall rate constant associated with both nucleation and growth rate parameters. Figure 9 shows the double logarithmic plots of log[− ln(1 − X(t))] versus log t for completely and partially melted PEEK samples crystallized at indicated crystallization temperatures. Each curve has a linear portion, most of which is followed by a gentle deviation at longer time. Usually, this deviation is considered to be due to the secondary crystallization, which is caused by the spherulite impingement in the later stage. By fitting the linear portion, the values of n and k can be obtained, which are included in Table 1. For PEEK without self-nucleation, the value of n ranges from 3.2 to 3.6 at T c = 320–325 °C (with the exception that n = 5.1 at T c = 327 °C), whereas it is from 2.4 to 2.8 at T c = 324–330 °C for the self-nucleated one. Obviously, the value of n for partially melted PEEK is lower than that of the completely melted one, indicating that self-nucleation does change the crystallization mechanism of PEEK. Similar conclusions have to be drawn for iPP [8, 39]. Moreover, it is reasonable to conclude that the crystallization of PEEK without self-nucleation is a homogeneous nucleation mechanism, while the one with self-nucleation is a heterogeneous nucleation mechanism.
Conclusions
The influence of pre-crystallization conditions on the self-nucleation behavior of PEEK has been investigated. Samples that were pre-crystallized nonisothermally or from the glassy state exhibit a larger self-nucleation effect than the ones pre-crystallized isothermally or from the melt. Moreover, an interesting finding is that the self-nucleation temperature domain of PEEK is way outside the melting curve, and its magnitude is greatly large (28–35 °C). The Avrami exponent for partially melted PEEK is lower than that of the completely melted one, indicating that self-nucleation changes the crystallization mechanism of PEEK from homogeneous nucleation to heterogeneous one.
References
Reiter G. Some unique features of polymer crystallization. Chem Soc Rev. 2014;43:2055–65.
Mamun A, Umemoto S, Okui N, Ishihara N. Self-seeding effect on primary nucleation of isotactic polystyrene. Macromolecules. 2007;40:6296–303.
Xu J, Ma Y, Hu W, Rehahn M, Reiter G. Cloning polymer single crystals through self-seeding. Nat Mater. 2009;8:348–53.
Fillon B, Lotz B, Wittmann JC. Self-nucleation and enhanced nucleation of polymers. Definition of a convenient calorimetric “efficiency scale” and evaluation of nucleating additives in isotactic polypropylene (α phase). J Polym Sci Polym Phys. 1993;31:1395–405.
Fillon B, Wittmann JC, Lotz B, Thierry A. Self-nucleation and recrystallization of isotactic polypropylene (α phase) investigated by differential scanning calorime. J Polym Sci Polym Phys. 1993;31:1383–93.
Lorenzo AT, Arnal ML, Sánchez JJ, Müller AJ. Effect of annealing time on the self-nucleation behavior of semicrystalline polymer. J Polym Sci Polym Phys. 2006;44:1738–50.
Xu Y, Wang Y, Xu T, Zhang J, Liu C. Crystallization kinetics and morphology of partially melted poly(lactic acid). Polym Test. 2014;37:179–85.
Li X, Su F, Ji Y, Tian N, Lu J, Wang Z, Qi Z, Li L. Influence of the memory effect of a mesomorphic isotactic polypropylene melt on crystallization behavior. Soft Matter. 2013;9:8579–88.
Xu T, Wang Y, He D, Xu Y, Li Q, Shen C. Nucleation effect of layered metal phosphonate on crystallization of isotactic polypropylene. Polym Test. 2014;34:131–9.
Xu T, Wang Y, Xu Y, Cao W, Liu C, Shen C. Crystallization behavior and nucleation analysis of isotactic polypropylene with a multiamide nucleating agent. Polym Test. 2014;36:62–8.
Supaphol P, Lin JS. Crystalline memory effect in isothermal crystallization of syndiotactic polypropylenes: effect of fusion temperature on crystallization and melting behavior. Polymer. 2001;42:9617–26.
Maus A, Hempel E, Thurn-Albrecht T, Saalwächter K. Memory effect in isothermal crystallization of syndiotactic polypropylene—role of melt structure and dynamics? Eur Phys J E. 2007;23:91–101.
Schneider S, Drujon X, Lotz B, Wittmann JC. Self-nucleation and enhanced nucleation of polyvinylidene fluoride (α-phase). Polymer. 2001;42:8787–98.
Supaphol P, Spruiell JE. Crystalline memory effect in isothermal crystallization of syndiotactic polypropylene. J Appl Polym Sci. 2000;75:337–46.
Wang Y, Mano JF. Influence of melting conditions on the thermal behaviour of poly(l-lactic acid). Eur Polym J. 2005;41:2335–42.
Martinelli A, Calì M, D’llario L, Francolini I, Piozzi A. Effect of the nucleation mechanism on complex poly(l-lactide) nonisothermal crystallization process. Part 1: thermal and structural characterization. J Appl Polym Sci. 2011;121:3368–76.
Attwood TE, Dawson PC, Freeman JL, Hoy LRJ, Rose JB, Staniland PA. Synthesis and properties of polyaryletherketones. Polymer. 1981;22:1096–103.
Zalaznik M, Kalin M, Novak S. Influence of the processing temperature on the tribological and mechanical properties of poly-ether-ether-ketone (PEEK) polymer. Tribol Int. 2016;94:92–7.
Leonardi A, Dantras E, Dandurand J, Lacabanne C. Dielectric relaxations in PEEK by combined dynamic dielectric spectroscopy and thermally stimulated current. J Therm Anal Calorim. 2013;111:807–14.
Yang Y. Sensitivity of nanoindentation strain rate in poly(ester-ester-ketone) using atomic force microscopy. Polym Test. 2016;53:85–8.
Hamdan S, Swallowe GM. Crystallinity in PEEK and PEK after mechanical testing and its dependence on strain rate and temperature. J Polym Sci Polym Phys. 1996;34:699–705.
Sarasua JR, Remiro PM, Pouyet J. Effects of thermal history on mechanical behavior of PEEK and its short-fiber composites. Polym Compos. 1996;17:468–77.
Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part 1. Crystallinity and interface adhesion. Compos Part A. 2000;31:517–30.
Bessard E, Almeida OD, Bernhart G. Unified isothermal and non-isothermal modelling of neat PEEK crystallization. J Therm Anal Calorim. 2014;115:1669–78.
Lin J, Ball J, Bremner T, Su HJ. Crystallization behavior and morphological characterization of poly(ether ether ketone). Polymer. 2014;55:5255–65.
Tardif X, Pignon B, Boyard N, Schmelzer JWP, Sobotka V, Delaunay D, Schick C. Experimental study of crystallization of polyetheretherketone (PEEK) over a large temperature range using a nano-calorimeter. Polym Test. 2014;36:10–9.
Rong C, Ma G, Zhang S, Song L, Chen Z, Wang G, Ajayan PM. Effect of carbon nanotubes on the mechanical properties and crystallization behavior of poly(ether ether ketone). Compos Sci Technol. 2010;70:380–6.
Nogales A, Ezquerra TA, Denchev Z, Baltá-Calleja FJ. Induction time for cold crystallization in semi-rigid polymers: PEN and PEEK. Polymer. 2001;42:5711–5.
Tierney JJ, Gillespie JW Jr. Crystallization kinetics behavior of PEEK based composites exposed to high heating and cooling rates. Compos Part A. 2004;35:547–58.
Liu T, Mo Z, Wang S, Zhang H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym Eng Sci. 1997;37:568–75.
Seo Y, Kim S. Nonisothermal crystallization behavior of poly(aryl ether ether ketone). Polym Eng Sci. 2001;41:940–5.
Bas C, Grillet AC, Thimon F, Albérola ND. Crystallization kinetics of poly(aryl ether ether ketone): time-temperature-transformation and continuous-cooling-transformation diagrams. Eur Polym J. 1995;31:911–21.
Tan S, Su A, Luo J, Zhou E. Crystallization kinetics of poly(ether ether ketone) (PEEK) from its metastable melt. Polymer. 1999;40:1223–31.
Wang Y, Funari SS, Mano JF. Influence of semicrystalline morphology on the glass transition of semicrystalline poly(l-lactic acid). Macromol Chem Phys. 2006;207:1262–71.
Toda A, Androsch R, Schick C. Insights into polymer crystallization and melting from fast scanning chip calorimetry. Polymer. 2016;91:239–63.
Bassett DC. Polymer morphology: a guide to macromolecular self-organization. Macromol Symp. 2004;214:5–15.
Avrami M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J Chem Phys. 1940;8:212–24.
Avrami M. Granulation, phase change, and microstructure kinetics of phase change. III. J Chem Phys. 1941;9:177–84.
Zhu X, Li Y, Yan D, Fang Y. Crystallization behavior of partially melting isotactic polypropylene. Polymer. 2001;42:9217.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (11432003, 51573170).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, Y., Lin, Q. et al. Crystallization behavior of partially melted poly(ether ether ketone). J Therm Anal Calorim 129, 1021–1028 (2017). https://doi.org/10.1007/s10973-017-6229-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6229-0