Abstract
This work focuses on the thermal characterization of a calcium silicate-based material synthesized with different solid wastes (chamotte and marble) for use as thermal insulation material. Thermal and structural changes occurring during heating were accompanied by differential thermal analysis, thermogravimetric analysis, dilatometric analysis, open photoacoustic cell technique, X-ray diffraction (XRD), and scanning electron microscopy. An endothermic event at 823.2 °C was interpreted as decomposition of carbonates. An exothermic event around 900 °C is associated with the crystallization of calcium silicate phases mainly wollastonite. The themophysical properties of the calcium silicate-based material (thermal diffusivity, thermal conductivity, specific thermal capacity, and thermal effusivity) are influenced by the synthesis temperature. The thermal analysis results agree well with the XRD. The calcium silicate pieces presented low thermal conductivity values (0.227−0.376 W m−1 K−1). These results suggest that the calcium silicate-based materials produced essentially with chamotte and marble wastes has high potential to be used as thermal insulation construction material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal insulators are materials employed in buildings to primarily reduce the heat flow. In particular, a good thermal insulation material exhibits low thermal conductivity value in order to reduce the heat transmission. The main thermal insulation materials used in buildings are hydrocarbon polymers, asbestos, perlite, fly ash, wood, mineral fiber, fiber glass, refractories, cotton, cement, and calcium silicate [1–4], which differ in terms of thermal properties and cost.
Calcium silicate is one of the most widely used inorganic lightweight thermal insulation material. It is applied in a wide temperature range (40–950 °C) [5, 6]. Calcium silicates are in the SiO2–CaO system [7], with special emphasis on wollastonite. The usual route used for production of calcium silicate materials is based on solid-state chemical reactions at high temperature. In addition, different Si and Ca sources could be used to synthesize calcium silicate materials such as diatomaceous earth, silica, silica fume, colloidal silica, hydrated lime, and quick lime [8]. During the synthesis process, the Si and Ca sources undergo a series of chemical transformations, which are accompanied by thermal reactions and mass transfer process. The knowledge about the thermal reactions makes easier the control of the thermal and physical properties of the calcium silicate-based materials produced.
The Brazilian industry produces high amounts of solid waste materials, including chamotte and marble wastes. Chamotte waste is rich in SiO2 [9, 10], and is produced during the clay brick processing. Nowadays only a small amount is used, resulting in environment impact. The ornamental rock industry produces high amount of marble waste rich in CaO, which is potentially pollutant [11, 12]. Technological alternatives to disposal of these solid waste materials need to be found and reuse is an option that meets current environmental, economical, and social concerns.
This study focuses on the thermal and structural characterization of calcium silicate-based material synthesized with different solid wastes as alternatives Si and Ca sources. The thermal properties of the calcium silicate-based materials have been also determined. A wide range of techniques are used to evaluate the transformations and properties: differential thermal analysis (DTA), thermogravimetry (TG), dilatometric analysis, open photoacoustic cell technique (OPC), X-ray diffraction (XRD), and scanning electron microscopy (SEM/EDS).
Experimental
Materials
Calcium silicate material was prepared using chamotte and marble wastes. The chamotte waste sample (defective fired clay brick) was collected in a clay ceramic plant located in southeastern Brazil (Campos dos Goytacazes-RJ). The marble waste sample was collected in an ornamental rock-cutting plant located in southeastern Brazil (Cachoeiro do Itapemirim-ES). Table 1 gives the chemical compositions of the raw materials determined by using energy-dispersive X-ray spectrometer. Table 2 presents the particle size distribution and particle density data of the raw materials.
Methods
In this work two solid wastes were used to produce calcium silicate-based materials. The chamotte waste was used as source of silica (SiO2), and the marble waste was used as source of calcium oxide (CaO). Initially, the chamotte and marble waste samples were crushed to a particle size below 150 mesh (<106 µm, ASTM). Then, the samples were mixed and homogenized by using a laboratory mixer during 2 h in a molar ratio of SiO2:CaO (1:1) [13]. Finally, the chamotte/marble mixture was fired at 1000, 1050, and 1100 °C in a laboratory electrical kiln at 20 °C min−1 to obtain calcium silicate material via solid-state reactions. For each firing temperature, a soaking time of 24 h was applied.
X-ray diffraction analysis of the raw materials and calcium silicate materials were performed with a Shimadzu XRD-7000 conventional diffractometer, using monochromatic Cu-Kα radiation (λ = 0.154059 nm) at 40 kV and 40 mA. The scanning speed was set to 1.5° (2θ) min−1. The interpretation of the X-ray diffraction data was carried out by comparing the Bragg peak positions and intensities with reference data listed in the ICDD cards.
The morphology of the calcium silicate powder was observed using a Shimadzu SEM SSX-550 scanning electron microscope, coupled with an energy-dispersive spectroscopy equipment, at 15 kV after platinum coating.
TG/DTA of the chamote/marble mixture in a molar ratio of SiO2:CaO (1:1) was carried out with a Netzsch Instrument STA 409E. The measuring parameters are summarized in Table 3. Dilatometric analysis of the chamote/marble mixture was done with a Netzsch CIL 402C dilatometer within the 25–1100 °C temperature range using a heating rate of 10 °C min−1 under air atmosphere.
The thermal diffusivity (α) measurements at room temperature were done by the open photoacoustic cell technique (OPC) [14], using disk samples of 10 mm diameter and thickness between 350 and 450 µm of the calcium silicate materials. The thermal diffusivity was determined according with the model proposed by Rosencwaig and Gersho [15] of an optically opaque and thermally thick sample. The specific heat capacity (ρC p) measurements were done using the photothermal technique of temperature rise under continuous light illumination of the calcium silicate sample in vacuum [14]. The thermal conductivity (k) was determined according to k = αρC p, in which ρ is the material density. The thermal effusivity (ε) of the samples was determined according to ε = (kρC p)1/2.
Calcium silicate pieces with 25 mm in diameter and 10 mm in height were produced by pressing at 82 MPa, cured by immersing them in water (5 min per day for 1 week), and then dried at 110 °C for 48 h. The water absorption and apparent density of the silicate pieces were determined according to the ASTM C373 standard. The water suction was determined according to the UNE 67-031 standard. Microstructural analysis of the fracture surface of calcium silicate pieces was done by SEM using a Shimadzu SEM SSX-500 at 15 kV after platinum coating.
Results and discussion
Structural and morphological characterization
XRD patterns of the chamotte and marble waste samples are presented in Fig. 1. The marble waste presented peaks that are mainly characteristic of calcite (CaCO3; ICDD card: 05-0586), with a small amount of dolomite (CaMg(CO3)2; ICDD card: 05-0622). In the chamotte sample characteristic peaks of quartz (SiO2; ICDD card: 46-1045), illite ((K,H3O)Al2Si3AlO10(OH)2; ICDD card: 26-911), and hematite (Fe2O3; ICDD card: 33-0664) were identified. An amorphous band between 2θ = 10° and 30° could be observed. This is caused by the dehydroxylation of the kaolinite structure with formation of amorphous metakaolinite [16–18]. In fact, the chamotte used in this work is a solid waste material from fired clay brick produced with kaolinitic clays [19].
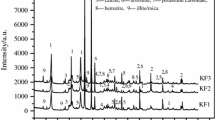
XRD patterns of the calcium silicate materials synthesized at different temperatures are shown in Fig. 2. Note that the chamotte/marble mixture in a molar ratio SiO2:CaO (1:1) underwent a series of phase transformations as temperature raised from room temperature up to 1000–1100 °C. The diffraction peaks of calcite, dolomite, quartz, illite, and hematite have disappeared during the synthesis process. The mineral phases identified in all synthesized materials are wollastonite (CaSiO3; ICDD card: 02-0689) as major calcium silicate material, and minor amounts of larnite (Ca2SiO4; ICDD card: 24-0037) and rankinite (Ca3Si2O7; ICDD card: 02-0323). The presence of ferrobustamite [(Ca,Fe,Mn)3Si3O9; ICDD card: 29-0336), gehlenite (2CaO.Al2O3.SiO2; ICDD card: 01-0982), anorthite (CaAl2Si2O8; ICDD card: 02-0523), and diopside (Ca,Mg(SiO3)2; ICDD card: 02-0656] has been also observed. A comparison of the XRD patterns indicates changes in the intensities of diffraction peaks with increasing synthesis temperature.
Figure 3 shows the morphological aspects of the synthesized calcium silicate powder. Figure 3a shows that the calcium silicate powder is composed mainly of irregular-shaped particles with a wide particle size range. The stacking of calcium silicate particles to form agglomerates is evident. The EDS analysis of the synthesized powder is shown in Fig. 3b. Ca, Si, O, Al, Mg, Fe, and C were detected. This is in accordance with the X-ray diffraction analysis (Fig. 2).
Thermal characterization
The TG/DTA curves of the chamotte/marble mixture in a molar ratio of SiO2:CaO (1:1) are shown in Fig. 4. Two endothermic events are seen in the DTA curve within the 150.0 and 823.2 °C temperature ranges, respectively. The first endothermic event concerns to the evolution of the physically adsorbed water on the solid waste particles. The second endothermic event observed at 823.2 °C is related to the carbonate decomposition to form mainly calcium oxide (CaO) and CO2 degassing. In fact, X-ray diffraction (Fig. 1) showed that the marble waste used is rich in calcite (CaCO3), with dolomite as the accessory mineral. This event was accompanied by an intense process of mass transfer in the chamote/marble mixture as shown in TG curve. The reactions related to the carbonate decomposition are [20–22]: (1) CaMg(CO3)2 → MgO + CaCO3 + ↑CO2; and (2) CaCO3 → CaO + ↑CO2. The chamotte/marble mixture presented a total mass loss of 18.14% during heating. An exothermic event around 900 °C could be observed. This event is probably related to a series of thermal reactions that could exist simultaneously and progressively in this temperature range to form new calcium silicate-based crystalline phases [7, 13, 23], mainly wollastonite given by CaCO3 + SiO2 → CaSiO3. This is in agreements with the X-ray diffraction results (Fig. 2).
The dilatometric curve of the chamotte/marble mixture in a molar ratio SiO2:CaO (1:1) is shown in Fig. 5. The dilatometric behavior involved in the chamotte/marble mixture could be described as follow. Initially, a slight expansion up to ~400 °C was observed, which is caused by the thermal expansion of the solid particles of the starting materials. Between ~400 and 500 °C a small inflexion occurred, which is mainly attributed to the α − β quartz transformation. A new expansion within the 500–800 °C range could be due to the carbonate decomposition to form mainly CaO and CO2 degassing. At ~838 °C a sharp shrinkage in the dilatometric curve was observed. Such behavior is associated with two events that take place simultaneously: the formation of calcium silicate phases via solid-state reactions and sintering. The shrinkage rate (dL/dt) reaches its maximum at 885.2 °C. These results are in accordance with the XRD (Fig. 2), SEM (Fig. 3) and TG/DTA (Fig. 4).
Figures 6–9 present the thermophysical properties of the calcium silicate materials synthesized at different temperatures. The thermal diffusivity is an important thermophysical property that measures how quickly heat propagates within a material. It is very sensitive to the material structure and synthesis conditions. The calcium silicate materials presented thermal diffusivity values within the 0.00389–0.00649 cm2 s−1 range (Fig. 6). These values are consistent with those for wollastonite (0.00270–0.00310 cm2 s−1) [24]. As shown in Fig. 7, the synthesized materials also presented low values of thermal conductivity (0.457–0.714 W m−1 K−1). The results in Fig. 6 also showed that the thermal diffusivity values were reduced with the increasing synthesis temperature. This lower thermal diffusivity reflects the change in the diffraction peak intensities as observed in the XRD data (Fig. 2). In fact, between 1000 and 1100 °C, a series of solid-state reactions are in progress to form new crystalline phases mainly calcium silicates (wollastonite, larnite, and rankinite). The thermal diffusivity behavior is quite correlated with all the other studied thermophysical properties. It could be seen that the thermal conductivity (Fig. 7), specific heat capacity (Fig. 8), and thermal effusivity (Fig. 9) tend to follow the thermal diffusivity behavior in relation to the synthesis temperature of the calcium silicate-based materials.
The thermal conductivity is considered to be the primary thermophysical property of a thermal insulation material. It denotes the efficiency of the material in the conduction of heat. In this context, calcium silicate-based material was produced in order to determine its application as thermal insulator. The structure of the ceramic pieces can be considered composed of a dense solid skeleton (synthesized calcium silicate material) and pores filled by air. The thermal conductivity (k m) of this two-phase system could be determined by the equation suggested by Kingery et al. [25] given by
in which k s is the thermal conductivity of the synthesized calcium silicate material and V p is the volume fraction of the porosity. Equation (1) is valid when the thermal conductivity of the solid skeleton (k s) is higher than that of the porous phase (air − k = 0.026 W m−1 K−1). The ceramic pieces presented porosity values within the 30.99–33.81% range.
The thermal conductivity values of the calcium silicate-based material pieces, as calculated by the Eq. (1), water absorption (WA), apparent density (AD) and water suction (WS) are presented in Table 4. As may be observed, the ceramic pieces presented small differences in the thermal conductivity values. This could be explained by the differences in the mineral phase composition as observed in the X-ray diffraction analysis (Fig. 2), apparent density, water absorption, and water suction of the produced ceramic pieces. In fact, SEM micrographs of the fractured surfaces of the ceramic pieces were compared to each other in Fig. 10. All pieces had a porous microstructure with only small differences in texture and porosity.
The calcium silicate-based material pieces exhibited thermal conductivity values within the 0.227–0.376 W m−1 K−1 range. According with the UNE-EN 1745 standard [26], the specified value of thermal conductivity for thermal insulation ceramic materials is lower than 0.430 W m−1 K−1. Thus, all calcium silicate-based material pieces presented thermal conductivity values lower than the reference value. The thermal conductivity of different insulation materials has been reported in the literature. It is in the (0.313–0.418 W m−1 K−1) range for insulation material of coal fly ash, perlite, clay, and linseed oil [2], (0.800–1100 W m−1 K−1) for clay building brick [27], and (0.300–3.000 W m−1 K−1) for kaolin bricks [28]. This means that the calcium silicate-based materials synthesized with solid wastes (chamotte and marble) via solid-state reactions has high potential to be used as thermal insulation material of high quality.
Conclusions
In this work, thermal and structural characterization of calcium silicate-based material produced with different solid wastes and synthesis temperature has been analyzed.
XRD analysis indicated that the marble waste used is rich in calcite, whereas the chamotte waste is rich in quartz. On heating, the chamotte/marble mixture in a molar ratio SiO2:CaO (1:1) underwent a series of solid-state reactions at high temperature, resulting in the formation mainly of wollastonite.
DTA curve has shown endothermic and exothermic events in the chamotte/marble mixture related to carbonate decomposition and phase transformations, respectively. The dilatometric behavior corroborates the DTA–TG and XRD results. It was found that the thermal properties are influenced by the synthesis temperature. The calcium silicate-based materials synthesized between 1000 and 1100 °C presented low values of thermal diffusivity and thermal conductivity.
It was found that all the calcium silicate-based material pieces presented values of thermal conductivity (0.227–0.376 W m−1 K−1) compatible with those specified for use as thermal insulation construction material.
References
Al-Homoud MS. Performance characteristics and practical applications of common building thermal insulation materials. Build Environ. 2005;40:353–66.
Balo F, Ucar A, Yucel HC. Development of the insulation materials from coal fly ash, perlite, clay and linseed oil. Ceram Silik. 2010;54:182–91.
Gertrude A. Thermal properties of selected materials for thermal insulation available in Uganda. Master Thesis. Uganda: Makerere University; 2011.
Al-Neafa M. Usability of PFBC-coal ash/glass wastes for heat insulators production. Int J Eng Technol. 2012;12:34–8.
Zheng Q, Chung SDL. Microporous calcium silicate thermal insulator. Mater Sci Technol. 1990;6:666–70.
Chi TD, Bentz DP, Stutzman PE. Microstructure and thermal conductivity of hydrated calcium silicate board materials. J Build Phys. 2007;31:55–67.
Taylor JR, Didsdale AT. Thermodynamic and phase diagram data for the CaO–SiO2 system. Calphad. 1996;14:71–88.
Krowl TR. Calcium silicate insulating material containing blast furnace slag cement. Patent US 6,869,475 B1; 2005.
Casagrande MC, Sartor MN, Gomes V, Della VP, Hotza D, Oliveira APN. Reaproveitamento de resíduos sólidos industriais: processamento e aplicação no setor cerâmico. Ceram Ind. 2008;13:34–42.
Gouveia FP, Sposto RM. Grog incorporation in ceramic mass for the manufacture of brick—a study of the physical–mechanical properties. Cerâmica. 2009;55:415–9.
Simsek C, Karaca Z, Gemici U, Gunduz O. The assessment of the impacts of a marble waste site on water and sediment quality in a river system. Fresenius Environ Bull. 2005;14:1013–23.
Saboya F, Xavier GC, Alexandre J. The use of the powder marble by-product to enhance the properties of brick ceramic. Constr Build Mater. 2007;21:1950–60.
Felipe-Sessé M, Eliche-Quesada D, Corpas-Iglesias FA. The use of solid residues derived from different industrial activities to obtain calcium silicates for use as insulating construction materials. Ceram Int. 2011;37:3019–28.
Mota L, Toledo R, Machado FAL, Holanda JNF, Vargas H, Faria RT Jr. Thermal characterisation of red clay from the northern region of Rio de Janeiro state, Brazil using an open photoacoustic cell, in relation to structural changes on firing. Appl Clay Sci. 2008;42:168–74.
Rosenbcwaig A, Gersho A. Theory of the photoacoustic effect with solids. J Appl Phys. 1976;47:64–9.
Kamsen E, Rizzuti A, Miselli P, Veronesi P, Leonelli L. Use of noncontact dilatometry for the assessment of the sintering kinetics during mullitization of three kaolinitic clays from Cameroon. J Therm Anal Calorim. 2009;98:757–63.
Wang H, Chunshan L, Peng Z, Zhang S. Characterization and thermal behavior of kaolin. J Therm Anal Calorim. 2011;105:157–60.
Belhouchet H, Chouia F, Hamidouche M, Leriche A. Preparation and characterization of anorthite and hydroxyapatite from Algerian kaolin and natural phosphate. J Therm Anal Calorim. 2016;126:1045–57.
Souza GP, Sanchez R, Holanda JNF. Characteristics and physical-mechanical properties of fired kaolinitic materials. Cerâmica. 2002;48:102–7.
Pleková E, Kozusníková A, Vaculíková C, Martynková GS. Thermal behavior of selected Czech marble samples. J Therm Anal Calorim. 2010;101:657–64.
Galan I, Glasser FP, Andrade C. Calcium carbonate decomposition. J Therm Anal Calorim. 2013;111:1197–202.
Szczes A, Sternik D. Properties of calcium carbonate precipitated in the presence of DPPC liposonres modified with the phospholipase A2. J Therm Anal Calorim. 2016;123:2357–65.
Kotsis I, Balogh A. Synthesis of wollastonite. Ceram Int. 1989;15:79–85.
Liu X, Ding C. Thermal properties and microstructure of a plasma sprayed wollastonite coating. J Therm Spray Technol. 2002;11:375–9.
Kingery WD, Bowen HK, Uhlmann DR. Introduction to Ceramics. 2nd ed. Hoboken: Wiley; 1976.
UNE-EN 1745, Masonry and masonry products – methods for determining thermal properties. 2013.
Kornann M. Clay bricks and roof tiles—manufacturing and properties. Paris: Société de L´industrie Minérale; 2007.
Michot A, Smith DV, Degot S, Goult C. Thermal conductivity and specific heat of kaolinite: evolution with thermal treatment. J Eur Ceram Soc. 2008;28:2639–44.
Acknowledgements
This study was supported by National Council for Scientific and Technological Development (CNPq). Authors also would like to thank the Cerâmica Sardinha for the supply of the chamotte waste and Moagem de Minérios Cachoeiro for the supply of the marble waste.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almeida, T.F., Leite, F.H.G., Faria, R.T. et al. Thermal study of calcium silicate material synthesized with solid wastes. J Therm Anal Calorim 128, 1265–1272 (2017). https://doi.org/10.1007/s10973-017-6100-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6100-3