Abstract
Thiram is one of the commonly used carbamate pesticides. Unfortunately, the thermal degradation and combustion of that substance can lead to emission of various toxic products that may cause a threat to humans and to the environment. In this study, the thermal degradation and combustion under air atmosphere were investigated. The experimental work consisted of thermogravimetric analysis using the STA–FT-IR and STA–GC–MS systems that ensure the simultaneousness of thermogravimetric analysis with the analysis of evolved gas. Moreover, the steady-state tube furnace (ISO TS 19700) has been used to generate gaseous products from real fires. The samples containing toxic products were collected using solid-phase microextraction technique and analyzed by GC–MS. The biggest number of gaseous products was emitted when the thermal degradation occurred at 350 °C. The comparison of FT-IR and GC–MS results led to identification of main toxic products such as sulfur dioxide, carbon disulfide, methyl isothiocyanate, tetramethylurea and tetramethylthiourea. All determined products are very dangerous substances. In particular, thioureas are carcinogenic, harmful and dangerous for the environment. Moreover, thioureas are alleged to be toxic to reproduction in humans and can cause harm to unborn children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thiram is a type of sulfur fungicides that belongs to the group of N,N-dialkyldithiocarbamate chemical pesticides [1]. It is used as a foliar treatment on fruits, vegetables and ornamentals to control Botrytis species, rust, scab and storage disease, and as a seed treatment to control seedling blights and a number of fungi that cause “damping off” in seedlings. Thiram formulations are registered for use in many countries [2]. In spite of benefits, thiram poses a potential threat to people and to the environment. Toxic products which can be released in fires or in the burning are one of the major threats.
Thermal decomposition and combustion of pesticide can occur in different situations, first of all during the processing of vegetables and foods poisoned by traces of pesticides [3–6]. Large amounts of hazardous substances such as pesticides are handled and stored every day in chemical plants and warehouses as a consequence of their massive use in the agricultural field [5, 7]. It has been reported in the past that fire occurred in a certain number of these installations involving large quantities of chemicals [8]. Toxicity, thermal instability and reactivity of pesticides caused several accidents not only during the storage of chemicals but also during the production and transport [9–11]. Another possibility of release of toxic species into the environment is the incorrect method of disposal of agricultural waste, for example burning of empty pesticide containers in open fires [12].
The products released during thermal decomposition and combustion of pesticides can cause serious air pollution, harmful not only to firefighters but also to people living in the surrounding area [13]. Moreover, water used by firefighting, mixed with these toxic products can contaminate the surface and ground water and cause major environmental pollution affecting the ecosystems [14]. The available literature related to thermal degradation of some pesticides [5, 15–24] reports different patterns of decomposition of compounds which depend on the temperature and the ratio of the mass of air to the mass of fuel during the combustion process. The incomplete combustion usually generates more toxic species mainly as a result of the fragmentation of the substances. Based on the published results, it can be concluded that heteroatom, such as sulfur, nitrogen, phosphorous, fluorine, bromide or chlorine present in the structures of pesticides, can convert into the large variety of toxic products during thermal degradation. The thermal decompositions of several dithiocarbamates have been studied by TG and DTG technique in air atmosphere. The decomposition processes have been followed by IR spectroscopy, but the obtained information is not sufficient. Authors noted that during the decomposition of thiram, compounds containing C–N, C=S and S–CS–N groups can be formed [25].
At the same time, sampling is perhaps the most critical part of procedures for analysis of gases in fire effluents. Whereas sampling and analysis are commonly used for many gaseous species in other fields, sampling from fire atmospheres presents unusual and difficult problems. The sample presented to the analyzer should be as representative as possible of the test atmosphere, without having been changed by the sampling system. Moreover, the sampling procedure should be as uncomplicated as possible and should be capable of operating with minimal blockage in the sampling lines, melting or other disruption of probes, and without allowing condensation of the species for analysis.
The solid-phase microextraction (SPME) technique has been widely used for the analysis of environmental pollutants in air, water, soil and sediment samples, using on-site or off-site analytical approaches [26]. In addition, SPME was successfully used to characterize compounds evolved during biomass pyrolysis [27].
The aim of this study is to evaluate the SPME sampling directly from the mixing chamber of Purser furnace in order to analyze the thermal degradation and combustion products of thiram. The steady-state tube furnace [28] has been used specifically to generate toxic products from real fires under different temperature conditions. The released species have been identified using gas chromatography with mass-selective detector (GC-MS). This work also presents information on the thermal degradation process occurring upon heating of thiram in air atmosphere. Experimental work consists of thermogravimetric (TG) experiments coupled with differential scanning calorimetry (DSC). Application of the simultaneous thermal analysis (STA) coupled online with Fourier transform infrared spectrometry (FT-IR) and with GC-MS allows to analyze the gaseous products.
Materials and methods
Materials
Tetramethylthiuram disulfide (thiram) 97% was purchased from Sigma-Aldrich Co. LLC. (Poland). The collection of analytes from tube furnace was performed with the use of a solid-phase microextraction manual holder supplied with 100-μm polydimethylsiloxane (PDMS) fiber, 75-µm Carboxen/polydimethylsiloxane (CAR/PDMS) fiber, and 65-µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber acquired from Supelco (USA).
Methods
Simultaneous thermal analyzer
Thermal degradation of thiram was studied in air atmosphere using the simultaneous thermal analyzer (STA 449F3 Jupiter, Netzsch, Germany). The 15-mg samples were placed in aluminum oxide crucibles and heated at the rate of 10 °C min−1 from room temperature (25 °C) to 900 °C. The flow rate of the air was 30 mL min−1, and the nitrogen was 20 mL min−1. To ensure the good reproducibility of the process, the experiments were performed at least three times.
Thermogravimetric analyzer: Fourier transform infrared spectroscopy–gas chromatography–mass spectrometry
The simultaneous thermal analyzer coupled with the Fourier transform infrared spectroscopy (Bruker Tensor 27 FTIR, Germany) (STA–FT-IR) and the gas chromatograph with a mass spectrometry (Agilent Technologies 7890 A GC MSD 5975, USA) (STA–GC–MS) was utilized to study the formation of volatiles produced in the thermal degradation process.
The FT-IR instrument was linked to the STA instrument through polytetrafluoroethylene pipe and flow cell, which were heated to 200 °C to prevent condensing of the released gases. After the evolved gases of samples from STA went through the flow cell, absorbance information was obtained at different wavenumbers as a function of temperature. The scanning range was from 4000 to 600 cm−1 at a resolution of 4 cm−1.
The GC–MS instrument was also linked to the STA instrument through a stainless pipe and with a pneumatic, needle-free injector. The transfer line and GC injector temperatures were maintained at 200 °C and 250 °C, respectively. The chromatographic separation was achieved with an HP-5 MS fused silica capillary column (30 m × 250 μm × 0.25 μm film thickness) from Agilent Technologies (USA). The oven temperature was initially maintained at 40 °C for 10 min and then increased to 250 °C at a heating rate of 10 °C min−1. Helium at a constant flow rate of 1 mL min−1 was used as the carrier gas, and the split ratio was 10:1. The separated compounds were then analyzed by the mass spectrometer, which was operated in electron ionization (EI) mode at the ionization energy of 70 eV. The mass spectra were obtained from m/z 15 to 350. Chromatographic peaks were identified through comparing the mass ions of each peak with NIST MS Library. On the basis of the NIST library, the highest possibility of product identification was chosen.
Steady-state tube furnace–gas chromatography–mass spectrometry
Thiram samples (10 g) in special test specimen boats were delivered into a furnace tube set at 25 °C. Then, the samples were heated to 900 °C with air flow 20 L min−1. The requirement in each test run was to obtain stable, steady-state decomposition conditions for at least 5 min during which the concentrations of effluent gases can be measured. Therefore, when the furnace temperature reached 150, 250, 350, 600 and 900 °C, it was maintained for 5 min to enable to collect the samples of fire effluent. The samples of effluent were taken from the mixing chamber of tube furnace by introducing the SPME device with the fiber to sampling port. After introducing the SPME syringe to the mixing chamber, the gaseous products of thermal decomposition of thiram were sorbed on the SPME fibers. After 5-min sorption, the fiber was withdrawn from the chamber and desorbed immediately in the GC injector for analysis. The operating conditions of GC–MS were the same as described above. The desorption time was 30 min. The fibers were conditioned in the injection port before use, according to the manufacturer’s instructions.
The chromatographic peak area of a specific compound is correlated linearly with its quantity, and its concentration can be reflected by the peak area ratio. The summed identified peak areas were normalized to 100%, and the relative abundance of specific compound can be reflected by its peak area ratio.
Results and Discussion
Simultaneous thermal analysis
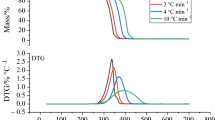
The thermogravimetric analysis (TG), differential thermogravimetric analysis (DTG) and differential scanning calorimetry (DSC) curves of thermal degradation of the thiram are shown in Fig. 1.
The thermal degradation of thiram is a multi-stage process, starts at about 154.3 °C and is completed at 332 °C. The main loss stage takes place in a temperature range from 200 to 280 °C. Then, tetramethylthiuram disulfide begins to degrade with lower thermal degradation rate. The mass loss is related to the emission of gaseous molecules; thiram does not leave residue.
STA–FT-IR and STA–GC–MS analysis
The 3D FT-IR spectra of the evolved gases from thiram thermal degradation process are presented in Fig. 2. According to the infrared spectra, the change in spectral intensity with temperature direction is the same as TG results. It can be noticed that most of the gases evolved between 180 and 260 °C. The released gasses are mainly composed of carbon disulfide (in wavenumber about 1538 cm−1). Selected FT-IR spectra of the thermal degradation of gaseous compounds were chosen for analysis at different temperatures. The spectra obtained at 185, 220, 260 and 330 °C are shown in Fig. 3.
When the temperature is 185 °C, the absorbance intensity of emitted products is weak and is shown in wavenumbers 1538 and 2072 cm−1. The strong band near 1538 cm−1 is probably related to the release of CS2, and it is related to the asymmetric stretch. Unfortunately, a sharp weaker band near 400 cm−1 which can confirm the presence of CS2 is out of the measure range. Because that band has no regular structure (there are inflections), it is possible that it can be a combination of several substances.
When the temperature of thermal degradation of thiram increases to 220 °C, the maximum absorbance intensity of carbon disulfide can be observed. In addition, in that temperature the new absorbance waves in the range 2800–3000 cm−1 are characteristic of carbon- and hydrogen-containing species and are assigned to various forms of C–H stretching. The bands below 1500 and at 1349 cm−1 can be assigned to asymmetric and symmetric deformation vibrations of CH3, respectively. The absorbance peaks at 2072 cm−1 can indicate the presence of asymmetric stretching vibration –N=C=S group characteristic of thioisocyanates. It is confirmed by the band presented at 1349 cm−1 (symmetric stretching vibration –N=C=S). Moreover, the gases evolved at 220 °C can contain –S–C≡N groups (the absorption peak at 2176 cm−1).
FT-IR spectra of thermal degradation products of thiram obtained at 260 °C are similar to spectra registered at 220 °C. One of the main differences is the intensity and shape of the absorption shown in wavenumber about 1500 cm−1. The structure of bands observed in that range supports the idea that they could be associated with several gaseous products obtained from the thermal degradation of the thiram. The absorption peak at 1524 cm−1 can be attributed to N–O asymmetric stretch characteristic of nitro compounds, or it can be related to =N–H stretching in secondary amine. The absorbance peak at 1125 cm−1 can indicate the presence of C–N stretch in tertiary amine. Moreover, the double peak in the range 1375–1345 cm−1 can indicate the presence of S=O asymmetric stretch [29, 30].
When the temperature of thermal degradation increases to 330 °C, it can be observed that absorbance intensiveness decreases. According to the results mentioned above, the thermal degradation of thiram in air atmosphere causes releasing of CS2, products containing –CH3, –N=C=S, –S–C≡N, C–N groups and probably products containing S=O and N–O groups. It can be concluded that the thermal degradation of thiram leads to formation of the small gaseous molecules as well as oxidation products.
The gaseous products of thermal degradation of thiram were investigated also by GC–MS method. The analyzed sample of gases was emitted at 206 °C, when the rate of mass lost was 14% min−1. The chromatogram obtained during analysis is shown in Fig. 4a. The main products of thermal degradation of thiram are CO2, SO2, CS2 and tetramethylthiourea. The information on main identified compound is presented in Table 1. Unfortunately, the concentration of the other compounds thermal degradation was not high enough to allow their detection and identification.
Total ion chromatograms from direct STA–GC–MS analysis (a) of gaseous compounds obtained during thermal degradation of thiram and SPME–GC–MS of gaseous products from thermal degradation of thiram in Purser furnace by different SPME fibers: CAR/PDMS (b), DVB/PDMS (c), PDMS (d). The numbers correspond to the products in Table 1
Steady-state tube furnace–gas chromatography–mass spectrometry analysis
The other way to investigate thermal degradation of emitted compounds of thiram is to use the steady-state tube furnace and GC–MS analysis.
The steady-state tube furnace used in investigation was Purser furnace [28]. It has been developed specifically to replicate the generation of toxic products from real fires under different fire conditions. Steady-state burning is achieved by delivering the sample into a furnace of increasing heat flux at a fixed rate and recording the product yields over a steady-state period of the run [31]. One of the main elements of that furnace is a mixing chamber where the compounds produced during combustion of thiram can react with each other and form other products.
The results obtained from TG/DSC curves show the temperatures where the significant degradations of thiram occur, which are accompanied by the emission of volatile products. Therefore, the temperatures: 150, 250 and 350 °C, were selected for the tests. Moreover, the samples of fire eluents were collected when the furnace temperature increased to 600 and 900 °C.
The samples containing the gaseous products of thermal degradation of thiram were collected using solid-phase microextraction. SPME is a technique that combines sampling and concentrating of analytes as well as introducing them to the chromatographic system [32]. Because released thermal decomposition products of thiram are composed of many compounds which have different polarity and volatility, the tests were carried out with the use of three types of SPME fibers. There were 65-µm polydimethylsiloxane/divinylbenzene (PDMS/DVB)-coated fiber—ideal for many polar analytes, especially amines; 75-µm Carboxen/polydimethylsiloxane (CAR/PDMS)-coated fiber—recommended for the extraction of polar and nonpolar volatile analytes; and 100-µm polydimethylsiloxane (PDMS)-coated fiber, ideal for low molecular weight or volatile compounds [33]. The chromatograms obtained during analysis of evolved gases during thiram thermal degradation at 350 °C using different types of SPME fibers are presented in Fig. 4b–d. The quantitative data concerning released compounds during thermal degradation of thiram are presented in Table 1.
The SPME sensitivity and selectivity depended mainly on the value of the partition coefficient for analytes between the fiber coating and the sample matrix, and hence depended on the type of stationary phase, the polarity and thickness of the fiber. Figure 4 shows that the number of identified compounds is higher when Carboxen/PDMS fiber coating is used. CAR/PDMS fiber has much better extraction efficiency than the other fibers; thus, it was selected as optimal and used in future studies.
In the next part of the study, the CAR/PDMS fiber was used to sampling the eluents from thermal degradation of thiram at different temperatures. The obtained results are presented in Table 2. Generally, during thermal decomposition of thiram at 150 °C the main emitting product is carbon dioxide. When the process of thermal decomposition takes place in 250 °C, the sample of thiuram begins to release two main products: carbon disulfide and tetramethylthiourea, and the yields of them are 32 and 59%. Moreover, in this condition the tetramethylurea and the small amounts of other substances were present in furnace atmosphere. The highest number of thermal degradation products of thiram is eluted at 350 °C. The major compounds are as follows: tetramethylthiourea, tetramethylurea, methyl isothiocyanate, N,N-dimethylformamide, carbon disulfide and N,N-dimethylthioformamide. Along with a further increase in temperature of thermal degradation, the number of formed compounds decreases. At 900 °C, the major compounds are carbon dioxide and tetramethylthiourea.
Conclusions
Thermal degradation process of thiram was studied using STA–FT-IR, STA–GC–MS and Purser furnace–GC–MS. The concentration and type of volatile products released during combustion of thiram depended on temperature of reaction. Generally, the thermal degradation of thiram leads to formation of the small gaseous molecules as well as oxidation products. The biggest number of products is formed when the thermal degradation occurs at 350 °C. The major compounds areas follows: tetramethylthiourea, tetramethylurea, methyl isothiocyanate, N,N-dimethylformamide, carbon disulfide and N,N-dimethylthioformamide, which are very dangerous substances.
Additionally, SPME technique can be successfully applied to sampling directly from the fire eluents in order to analyze thermal degradation and combustion products. SPME could represent a helpful tool for sampling and analysis that could be employed for monitoring the thermal degradation and combustion processes avoiding sample collection and sample pretreatment, thus reducing laboratory working time.
References
Hernández-Olmos MA, Agüí L, Yáñez-Sedeño P, Pingarrón JM. Analytical voltammetry in low-permittivity organic solvents using disk and cylindrical microelectrodes. Determination of thiram in ethyl acetate. Electrochim Acta. 2000;46:289–96.
Lohse R, Jakobs-Schönwandt D, Vidal S, Patel AV. Evaluation of new fermentation and formulation strategies for a high endophytic establishment of Beauveria bassiana in oilseed rape plants. Biol Control. 2015;88:26–36.
Holden AJ, Chen L, Shaw IC. Thermal stability of organophosphorus pesticide triazophos and its relevance in the assessment of risk to the consumer of triazophos residues in food. J Agric Food Chem. 2001;49:103–6.
Juhler RK. Supercritical fluid extraction of pesticides from meat: a systematic approach for optimization. Analyst. 1998;123:1551–6.
Senneca O, Scherillo F, Nunziata A. Thermal degradation of pesticides under oxidative conditions. J Anal Appl Pyrolysis. 2007;80:61–76.
Petkova V, Serafimova E, Kostova B. Thermal behaviour of nitric-acid-treated biomass and chicken litter mixtures. J Therm Anal Calorim. 2016;126:149–60.
Andreozzi R, Ialongo G, Marotta R, Sanchirico R. The thermal decomposition of dimethoate. J Hazard Mater. 1999;64:283–94.
Christiansen V. Combustion of some pesticides and evaluation of the environmental impact. J Loss Prev Process Ind. 1994;71:39–48.
Cozzani V, Smeder M, Zanelli S. Formation of hazardous compounds by unwanted reactions in industrial accidents. J Hazard Mater. 1998;63:131–42.
Koller G, Fischer U, Hungerbühler K. Assessing safety, health, and environmental impact early during process development. Ind Eng Chem Res. 2000;39:960–72.
Sanchirico R, Pinto G, Pollio A, Cordella M, Cozzani V. Thermal degradation of fenitrothion: identification and eco-toxicity of decomposition products. J Hazard Mater. 2012;199–200:390–400.
Damalas CA, Telidis GK, Thanos SD. Assessing farmers’ practices on disposal of pesticide waste after use. Sci Total Environ. 2008;390:341–5.
Smith-Hansen L, Jogensen KH. Characterisation of fire products from organophosphorus pesticide using the DIN 53436 method. J Loss Prev Process Ind. 1993;6:227–32.
Atkinson GT, Jagger SF. Exposure of organophosphorus pesticides to turbulent diffusion flames. J Loss Prev Process Ind. 1992;5:271–7.
Altarawneh M, Carrizo D, Ziolkowski A, Kennedy EM, Dlugogorski BZ, Mackie JC. Pyrolysis of permethrin and formation of precursors of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/F) under non-oxidative conditions. Chemosphere. 2009;74:1435–43.
Chen K, Mackie JC, Kennedy EM, Dlugogorski BZ. Determination of toxic products released in combustion of pesticides. Prog Energ Combust. 2012;38:400–18.
Kaisersberger E, Post E. Applications for skimmer coupling systems, combining simultaneous thermal analysers with mass spectrometers. Thermochim Acta. 1998;324:197–201.
Lubkowski J, Janiak T, Czerminski J, Blazejowski J. Thermoanalytical investigations of some chloro-organic pesticides and related compounds. Thermochim Acta. 1998;155:7–28.
Nageswara R, Khalid S, Rajani T, Husain S. Gas chromatographic-mass spectrometric separation and identification of combustion products of organo-phosphorus and chlorine pesticides and evaluation of their impact on the environment. J Chromatogr. 2002;954:227–34.
Summoogum SL, Mackie JC, Kennedy EM, Dlugogorski BZ. Formation of toxic species and precursors of PCDD/F in thermal decomposition of alpha-cypermethrin. Chemosphere. 2011;85:143–50.
Rodante F, Marrosu G, Catalani G. Thermal analysis and kinetic study of decomposition processes of some pesticides. J Therm Anal. 1992;38:2669–82.
Rodante F, Marrosu G, Catalani G. Thermal analysis and kinetic study of decomposition processes of some commercial pesticides I. Triazine derivatives. J Therm Anal Calorim. 1998;53:937–56.
Rodante F, Vecchio S, Catalani G, Guidotti M. Thermal analysis and non-isothermal kinetic study of some pesticides. Part II. Chlorinate derivatives. J Therm Anal Calorim. 2000;60:605–22.
Nunes AR, Moura AO, Prado AGS. Calorimetric aspects of adsorption of pesticides 2,4-d, diuron and atrazine on a magadiite surface. J Therm Anal Calorim. 2011;106:445–52.
Fernandez-Alba A, Perez-Alvarez IJ, Martinez-Vidal JL, Gonzalez-Pradas E. Thermal and spectroscopic study of several dithiocarbamates. Termochim Acta. 1992;211:271–7.
Ouyang G, Pawliszyn J. SPME in environmental analysis. Anal Bioanal Chem. 2006;386:1059–73.
Conti R, Fabbri D, Torri C, Hornung A. At-line characterization of compounds evolved during biomass pyrolysis by solid-phase microextraction SPME-GC-MS. Microchem J. 2016;124:36–44.
ISO/TS 19700. Controlled equivalence ratio method for the determination of hazardous components of fire effluents. Geneva: International Organization for Standardization; 2007.
Coates J. Interpretation of infrared spectra, a practical approach. In: Meyers RA, editor. Encyclopedia of analytical chemistry. Chichester: John Wiley&Sons Ltd.; 2000. p. 1–23.
Silverstein RM, Webster FX, Kiemle DJ, Bryce DL. Spectrometric identification of organic compounds. 8th ed. Hoboken: Wiley; 2015. p. 71–101.
Stec AA, Hull TR, Lebek K. Characterization of the steady state tube furnace (ISO TS 19700) for fire toxicity assessment. Polym Degrad Stabil. 2008;93:2058–65.
Mark P, Sandercock L. Fire investigation and ignitable liquid residue analysis—a review: 2001–2007. Forensic Sci Int. 2008;176:93–110.
Shirey RE. SPME commercial devices and fibre coatings. In: Pawliszyn J, editor. Handbook of solid phase microextraction. London: Elsevier; 2012. p. 99–132.
Acknowledgements
This paper was based on the results of a research task carried out within the scope of the third stage of the National Programme “Improvements of safety and working conditions” partly supported in 2014-2016—within the scope of state service—by the Ministry of Labour and Social Policy. The Central Institute for Labour Protection—National Research Institute was the Programme’s main coordinator.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sankowska, M., Gajek, A., Celiński, M. et al. Determination of gaseous products of thermal degradation of thiram. J Therm Anal Calorim 128, 1639–1647 (2017). https://doi.org/10.1007/s10973-016-6043-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6043-0