Abstract
Detergent–dispersant additized gasoline helps to keep engines clean, inhibits the formation of gum and sludge in the combustion process, and reduces the emissions of contaminants to the atmosphere. In this study, a new procedure using thermogravimetry (TG), derived thermogravimetry (DTG), and differential scanning calorimetry (DSC) was developed to identify the presence of four commercial detergent–dispersant additives used in gasoline in Brazil using sealed aluminum pans with small holes and pinholes and an inert ambient. It was found that when pinholes of the same diameter were used, it was possible to distinguish the additives more accurately. The thermal decomposition temperatures of the active components of each additive were mainly identified by TG and DTG. The results indicate that the active components of additives T and Y are similar, but that their content is slightly different. They can be rapidly and easily distinguished from additives W and G, which allows their quantification by size-exclusion chromatography in real samples of Brazilian gasoline to which they may have been added.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental concerns about gas emissions have driven improvements in the quality control of fuels. Following the example of other countries, including the USA, which in 1996 introduced the requirement for detergent additives to be added to gasoline to reduce emissions of pollutants from automotive vehicles, the Brazilian Oil, Gas and Biofuels Regulatory Agency (ANP) passed a regulation in 2013 (ANP Resolution 40/2013) that sets the specifications for gasoline for phase L-6 of the Automotive Vehicle Air Pollution Control Program (PROCONVE) and the rules for the use of detergent–dispersant additives in this fuel [1–3]. This resolution requires up to 5.000 mg kg−1 detergent–dispersant additives to be added to Brazilian gasoline as of July 2017, with a view to keeping engines clean and reducing the formation of gum and sludge during the combustion process [2–5].
The detergent additives act via polar terminal groups. They generally contain nitrogen, which adheres to metal surfaces, forming a protective film that prevents the formation of residues on the internal surfaces of engines. Dispersant additives contribute to the dispersion of the particles that enter the engine via the air, fuel, or lubricant, keeping them suspended as solids and finally dispersing them, thereby preventing the buildup of deposits [6–8]. The benefits of using these compounds include a 3% reduction in fuel consumption, a 60–70% reduction in deposit formation, a 50–60% reduction in carbon monoxide and hydrocarbon emissions, and a 20% reduction in nitrogen oxide emissions [1, 2].
According to Danilov [6], Colaiocco and Lattanzio [8], and Silin et al. [9], the active ingredients of detergent additives may include amines, amides, amine-amides, alkenyl succinimides, polybutene amines, or other compounds, dissolved in mineral oils.
As the composition of detergent–dispersant additives is often a trade secret, few methods for characterizing them were encountered in the literature. Pimentel et al. [2] have developed a method for identifying and quantifying the detergent–dispersant additives present in Brazilian gasoline (G, T, W, and Y, provided by Brazilian Oil, Gas and Biofuels Regulatory Agency (ANP)) that involves concentrating them using the ring oven technique and then using near-infrared hyperspectral imaging for their analysis. Santos [5] and Santos and d’Avila [4] have used size-exclusion chromatography to identify and quantify the detergent–dispersant additives G, T, and W, also provided by ANP, in Brazilian gasolines.
Colaiocco and Lattanzio [8] have described a method for identifying and quantifying amide detergents using size-exclusion chromatography. Patents CN1673737A, PI0400082-0A, and CA2132806A1 propose methods for separating and identifying detergent–dispersant additives in gasoline by thin layer chromatography and size-exclusion chromatography [10–12]. Pinto et al. [13] have used nuclear magnetic resonance spectroscopy of hydrogen (1H NMR) along with principal component analysis (PCA) and soft independent modeling of class analogies (SIMCA) to differentiate between common gasoline (CG) and additized gasoline (AG). The methodology allows to detect the presence of additives used in commercial gasolines and can be an important tool for quality control of the product. Thermoanalytical techniques can be used on a wide range of materials and are being employed in a broad range of applications thanks to the great potential they have demonstrated [14, 15].
This study used thermogravimetry (TG), derived thermogravimetry (DTG), and differential scanning calorimetry (DSC) to distinguish detergent–dispersant additives in real Brazilian gasoline samples. It is designed to determine the contents and specific thermal volatilization and/or decomposition temperatures of the active components and solvents used in commercial gasoline additives, mainly by TG and DTG, which are a function of their chemical composition and structure.
This novel method could be used in the quality control of gasoline additives and is a first step toward the development of new official monitoring procedures for additized gasoline quality control in Brazil and worldwide, as well as for future research into their quantification by size-exclusion chromatography, the method proposed by Santos and Santos and d’Avila. The complete characterization will also depend on the method being developed by the authors for the calculation of their respective concentrations in gasoline, since the quantity of active components and solvents differs in each commercial additive, as shown in this paper.
Materials and methods
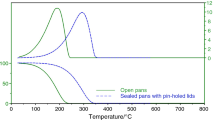
Four detergent–dispersant additives (G, T, W, and Y) provided by ANP were analyzed by TG, DTG, and DSC using a TA Instruments thermal analyzer, model Q600. Samples with masses of the order of 7 mg were placed in aluminum pans, which were then sealed with a lid that had a hole bored manually into it measuring around 0.5 mm in diameter. When these kinds of pans and lids are used, as the temperature increases the space above the liquid sample is progressively saturated by the gases of the more volatile components, which are only released to the external environment when their vapor pressure is practically equal to atmospheric pressure (101,325 N m−2), causing a corresponding mass loss, which can be measured by TG. The smaller the diameter of the hole, the closer to atmospheric pressure the vapor pressure of the accumulated gas must be to escape from inside the pan and the better the separation of the different gases released by volatilization or temperature decomposition (pyrolysis) of the components of a sample [14].
The mass loss rate at a specific temperature is measured by the respective DTG curve value, and different DTG peaks usually indicate the same product being volatilized or decomposed or, in the case of organics, a group of the same type of compounds with different molecular weights. The difference of the residual masses measured in the TG curves between the limits of the DTG peaks corresponds to the total mass lost during its occurrence. Usually, for a group of the same type of organic compounds, the higher the chain length and/or the molecular weight of the liquid component, the higher its respective volatilization or pyrolysis temperature, which can be used to compare qualitatively some of the characteristics of the components of a sample, when released in this way [14].
Thus, to improve the reproducibility of volatilization or decomposition temperatures of the same components, the analyses were also done in hermetically sealed Tzero pans (TA Instruments) with identical laser-cut pinholes of less than 0.1 mm in their lids. For all the analyses, the heating rate used was 10 °C min−1 from ambient temperature to 600 °C and the flow rate of the carrier gas, nitrogen, was 100 mL min−1. The TG, DTG, and DSC curves were obtained and analyzed using TA Instruments Universal Analysis data processing software.
Results and discussion
Figure 1 shows the TG, DTG, and DSC curves obtained for additive G, showing typical behavior for the four additives. All the results indicate the existence of two separate stages of mass loss at distinct temperature ranges. It seems that at the lower temperature range—up to about 300 °C—a mineral oil, which acts as a solvent, is being volatilized, while at a much higher temperature range, the active components or agents of the respective additives are volatilized and/or decomposed.
According to Danilov [6], Colaiocco and Lattanzio [8], and Silin et al. [9], the active ingredients of detergent additive packages may include amines, amides, amine-amides, alkenyl succinimides, polybutene amines, or other compounds, dissolved in mineral oils. This explains why mass was lost in two stages in the thermal analyses shown in Fig. 1 and indicates that the additives are different organic substances than the solvents, which are volatilized at a lower temperature range.
Figure 1 also shows that additive G’s first stage of endothermic mass loss occurred up to 298 °C, as seen in the respective DSC curve. The second stage, also endothermic, occurred between 300 and 500 °C, which was due to the thermal decomposition of the fraction with a higher molecular weight, corresponding to the active component of the additive, as this has a high molecular weight. According to Santos and d’Avila [4], Danilov [6], and Colaiocco and Lattanzio [8], the active components of the additives have a higher molecular weight, as confirmed by their thermal decomposition curves. It is important to note that this was seen in all the thermal decomposition TG and DTG curves of the additives under analysis.
It can be observed from Fig. 1 that the active component of additive G is stable at high temperatures and the same goes for the other three additives. According to Danilov [6] and Silin et al. [9], the latest multifunctional additives have to meet strict requirements; in particular, they must show high thermal stability to allow for effective engine operation at temperatures up to 200–300 °C.
In Fig. 2, the TG and DTG curves of all the additives tested are superimposed for comparative purposes. The active components of additives G and W began their mass loss at similar temperatures (287 and 298 °C), but at a lower temperature, than samples T and Y, which started to lose mass at practically the same temperature (312 and 313 °C). The TG curves of the active components of additives T and Y look similar, but their DTG curves do not, due to different mass losses attributed to solvents. Santos and d’Avila found that additive T behaved differently from additives W and G, using the atmospheric distillation technique for the pre-concentration of the additives followed by analysis by size-exclusion chromatography with a conventional refractive index detector, while Pimentel et al. found the same similarities using near-infrared spectroscopy with the partial least-squares model [2, 4].
As was discussed previously, in order to compare the temperature ranges at which each stage of mass loss took place more easily and accurately (especially the active components, to identify whether they were the same or not), the analyses were repeated under the same operational conditions, but using Tzero pans (TA Instruments) hermetically sealed with identical laser-cut pinholes of less than 0.1 mm in their lids. The new TG and DTG curves for additives G are shown in Fig. 3 and superimposed against the earlier results. With the smaller pinhole diameter, mass losses occurred at higher temperature ranges and, according to their respective DSC curves, with higher enthalpies of transformation.
With a smaller pinhole, the internal pressure is higher, raising the volatilization and cracking temperatures of the additive components. The pans with identical pinholes also provided identical operational conditions and therefore more reliable comparative data, which was not the case of the pans with manual pinholes, as shown in Fig. 2 [14, 15]. Therefore, the pans with identical pinholes were used in the next experiments and will be used in future research, since they provide greater accuracy and precision of results.
Figure 4 shows the TG and DTG curves for additives G, T, W, and Y obtained using hermetic aluminum Tzero pans with a laser-cut pinhole in the lid. It can be seen that the DTG peaks relating to the mass loss of the active ingredients in additives T and Y are the same, but that their content is slightly different, as shown in the respective TG curves. For additives W and G, the mass loss attributed to the active components is slightly different, showing again the similarity between additives W and G and between additives T and Y, by thermogravimetry, as Santos and d’Avila and Pimentel et al. previously shown. It indicates that additives W and G have a similar chemical composition and can be distinguished from additives T and Y. The investigation of the chemical properties of these additives was not the purpose of this paper, which was only designed to develop a procedure to easily distinguish them.
Conclusions
-
The thermal analyses of the additives using hermetic aluminum pans with identical and lowest pinhole diameters enabled a better separation of the stages of mass loss of the solvents and active ingredients present in the additives.
-
The use of identical laser-cut pinholes of a smaller diameter than the handmade ones, whose sizes also varied slightly, permitted the analysis and separation of the components to be performed under identical conditions, enabling a more accurate comparison of the additives’ respective components.
-
The active components of additives T and Y are similar, but their content is slightly different. They can be rapid and easily distinguished from additives W and G by using the developed procedure, which allows their quantification by size-exclusion chromatography in commercial Brazilian gasoline samples, to which they may have been added.
References
Fedorova AV, Krylov IF, Emel’yanov VE, Nikitina EA. Detergent additives to automotive gasolines. Chem Technol Fuels Oils. 2006;42:1.
Pimentel MF, Honorato FA, Pasquini C, Rohwedder JJR, Silva MPF, Brito LR. Determination of detergent and dispersant additives in gasoline by ring-oven and near infrared hypespectral imaging. Anal Chim Acta. 2015;863:9.
ANP Resolution Nº40. 2013. http://www.anp.gov.br. Accessed 08 Apr 2016.
Santos APF, d’ Avila LA. Análise de aditivos detergentes/dispersantes em gasolinas por Cromatografia de exclusão por tamanho [Analysis of detergent/dispersant additives in gasolines by size-exclusion chromatography]. International Symposium of Automotive Engineering (SIMEA). 2012.
Santos APF. Desenvolvimento de métodos para separação e identificação semiquantitativa do biodiesel e/ou óleo vegetal no óleo diesel e de aditivos em gasolina [Development of methods for the separation and semi-quantitative identification of biodiesel and/or vegetable oil in diesel and additives in gasoline]. Master’s Dissertation. Escola de Escola de Química da Universidade Federal do Rio de Janeiro. 2012.
Danilov AM. Progress in research on fuel additives (review). Pet Chem. 2015;55(3):169.
Medeiros ARB. Uso de ATR/FTIR e FTNIR associado à técnicas quimiométricas para quantificação de aditivos em gasolina automotiva [Use of ATR/FTIR and FT-NIR associated with chemometric techniques for the quantification of additives in automotive gasoline]. Master’s Dissertation. Instituto de Química da Universidade de Brasília. 2009.
Colaiocco S, Lattanzio M. Determination of additives in gasoline by SEC coupled to a light-scattering detector—chemometrics application. J High Resolut Chromatogr. 1995;18:397.
Silin MA, Ivanova LV, Burov EA, Koshelev VN, Bordubanova EG. Synthesis and testing of polyalkenyl succinimides as components of detergent additives for motor fuels. Pet Chem. 2012;52:272–4.
Mingxing F, Guoqing L, Lili W. Patent CN 1673737A entitled “Method for separating and measuring cleaning agent in gasoline”. 2004.
Fedorova GV. Patent PI 0400082-0A entitled “Método analítico para detecção e quantificação de aditivos de combustível” [Analytical method for the detection and quantification of fuel additives]. 2004.
Brauer SJ, Miin TT. Patent CA 2132806A1 entitled “Method for quantitatively determining detergent fuel additives in fuel samples”. 1994.
Pinto VS, Gambarra-Neto FF, Flores IS, Monteiro MR, Lião LM. Use of 1H NMR and chemometrics to detect additives present in the Brazilian commercial gasoline. Fuel. 2016;182:27.
Dweck J. Fatores experimentais importantes em termogravimetria [Significant experimental factors in thermogravimetry]. Internal Publication of Thermal Analyses Course of Materials and Processes. Escola de Química da Universidade Federal do Rio de Janeiro. 2015.
Lonashiro M, Caires FJ, Gomes DJC. Giolito. Fundamentos da Termogravimetria e Análise Térmica Diferencial/Calorimetria Exploratória Diferencial [Fundamentals of Thermogravimetry and Differential Thermal Analysis/Differential Scanning Calorimetry]. 2nd ed. São Paulo:Giz Editorial; 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
dos Santos, A.P.F., da Silva, K.K., d’ Avila, L.A. et al. Characterization of fuel detergent–dispersant additives by thermogravimetry. J Therm Anal Calorim 131, 783–788 (2018). https://doi.org/10.1007/s10973-016-6035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6035-0