Abstract
Since many polymer–organoclays nanocomposites are prepared in melt state, the thermal stability of the surfactants present in these organoclays is extremely important. Alkyl ammonium salts are surfactants which have been used in organoclays, but the low thermal degradation temperature of these salts is a drawback for polymer nanocomposites preparation in the melt state at temperatures higher than 200 °C. In order to obtain organoclays more suitable to be used in these polymer nanocomposites, in this work clay minerals were modified with more thermally stable organic modifiers than conventional salts. Two types of commercial clay minerals were organically modified with alkyl ammonium salt, alkyl and aryl phosphonium salts and an organosilane compound. X-ray diffraction, thermogravimetric analysis (TG) and infrared analysis results indicate that for both commercial clay minerals the preparation of the organoclays was efficient. TG analysis confirmed that phosphonium and silane organoclays are more thermally stable as compared with conventional alkyl ammonium organoclays. It was also observed that the thermal resistance of the organoclay depends on the type of the aluminosilicate used for the organic modification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Layered silicates or clay minerals are widely used as thixotropic components in fluids of petroleum well perforation, in metal foundry industries, lubrificants, paints, adhesives and cosmetics [1, 2] as well as in water purification [3–5] and as catalysts and catalytic support for organic reactions [6]. In recent years, layered silicates have been also used as nanofillers for various polymer matrices, forming the so-called polymer–clay mineral nanocomposites. There have been large interest in these nanocomposites because a small amount of organoclay (about 5 mass%) can lead to a large improvements in various properties of the polymer matrix [7–13]. In order to improve the compatibility between hydrophilic clay minerals and the organophilic polymers, the interlayer cations should be replaced by organic cations through an ionic exchange reaction, leading to the formation of organoclays. Depending on the chemical structure, packing density and molecular volume of these surfactants, the enthalpy and entropy of mixing of the organoclay with the polymer can be optimized in order to promote intercalation of polymer chains inside the clay mineral galleries and eventually lead to truly nanocomposites formation, where individual clay mineral platelets are dispersed in the polymer matrix [14–16].
Quaternary ammonium salts are compounds frequently used as surfactants in organoclays because they can be easily exchanged with ions situated between the layers of clay minerals and also because of its low price. However, the main drawback of these compounds is the low thermal decomposition temperature, around 180 °C [17]. This is a concern for polymer–organoclay nanocomposites obtained by melt compound, since the melt temperature of most synthetic polymers is higher than this temperature and therefore the organic salt can be decomposed during the nanocomposite melt processing. This decomposition by its turn could lead to clay mineral platelets collapse and worsening of the compatibility between clay mineral and polymer due to the alteration of the interface clay mineral/polymer, precluding the formation of nanocomposites [18]. Moreover, the products from the thermal degradation of the organic salt are likely to accelerate the thermal degradation of the polymer chains of nanocomposites [19, 20].
In order to avoid the thermal decomposition of the organic salt during the nanocomposite processing, others organic salts having higher decomposition temperatures than alkyl ammonium salts have been considered as organic intercalants for the montmorillonite. Among these salts, it should be highlighted the ionic liquids. These compounds can be produced with a variety of cations and anions. More common organic cations are derivated of imidazolium, pyridinium and phosphonium [21–27]. Besides the high thermal resistance, it is also possible to control the hydrophilic/lipophilic balance of the ionic liquids structure across proper selection of the constituting ions.
Smectite clay minerals modified with organosilanes are another type of organoclays which usually present high thermal decomposition temperatures. In this case, the organic modification can take place by the following ways: adsorption of the organosilanes into the clay mineral galleries (intercalation), adsorption on the clay mineral external surface and covalent bond of the organosilane on the internal surface of clay mineral platelets or onto the edge side of the clay mineral [28–34]. This last type of modification occurs due to the silylation reaction between alkoxysilane groups from the organosilane and the hydroxyl groups which are present on the edge of smectite clay minerals [35, 36].
In this work, it was prepared and characterized three organoclays with two phosphonium ionic liquids and one silane compound in order to compare their thermal properties with a typical alkyl ammonium organoclay. Moreover, the organoclays were prepared by using two commercial montmorillonites with different cation-exchange capacity (CEC), in order to evaluate how the properties of organoclays are affected by the type of inorganic hosts.
Experimental
Materials
Montmorillonite Cloisite® Na+ was purchased from Southern Clay, USA, and montmorillonite Nanomer® Na+ was purchased from Nanocor, USA. According to suppliers, the cationic exchange capacity of these clay minerals is 92 meq/100 g and 145 meq/100 g, respectively. In this work, we used the acronyms Mt-1 and Mt-2 for montmorillonites Cloisite Na+ and Nanomer Na+, respectively. Montmorillonite Mt-1 30B was purchased from Southern Clay, USA, for comparison with others organoclays after the modification.
Organic modifiers used to the organophilization of the montmorillonites were:
-
1.
Tetradecyl(trihexyl)phosphonium bromide (Cytec Industries), molar mass = 483.85 g mol−1; acronym: PHOSPHONIUM 1;
-
2.
Butyl(triphenyl)phosphonium bromide (Cytec Industries), molar mass = 319.40 g mol−1; acronym: PHOSPHONIUM 2;
-
3.
3-Aminopropylmethyldiethoxysilane YH-62, (Sigma-Aldrich), molar mass = 191.1 g mol−1; acronym: SILANE;
-
4.
Bis(2-hydroxy-ethyl)methyl tallow ammonium, which is the organic modifier of the commercial organoclay Mt-1 30B, from Southern Clay, molar mass = 359 g mol−1; acronym: AMMONIUM;
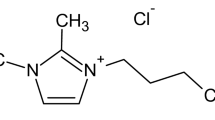
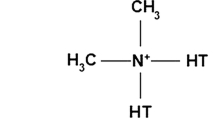
The chemical structures of these organic modifiers are illustrated in Fig. 1.
Illustration of the chemical structure of organic modifiers used in this work: a phosphonium 1, b phosphonium 2, c silane and d alkyl ammonium (from Mt-1 30B), where T = tallow is a natural products composed predominantly of octadecyl alkyl chains (65 %) [20]
Methods
Modification of montmorillonite clay minerals
Organoclays were obtained from mixing colloidal suspensions of Mt-1 or Mt-2 with solutions of phosphoniums or silane modifiers. For phosphonium 1 and silane, the solutions were prepared in distilled water and isopropyl alcohol at temperature between 60 and 80 °C. The ratio water:alcohol was 1:1 and 1:3 for the phosphonium 1 and silane, respectively. Phosphonium 2 was dissolved directly into distilled water at temperature between 60 and 80 °C. The concentration of the modifiers on water or water:alcohol was about 5 mass%. Mt-1 and Mt-2 clay minerals were separately dispersed in a separated suspension-containing distilled water and/or alcohol at a solid concentration of about 2 mass%. Next, the solution containing the modifiers was slowly added into this suspension. In the final suspension, the concentration of organic modifier relative to clay mineral was about 1.5 times the CEC of the clay mineral. This final suspension was maintained under mechanical stirring for 3 h and under temperature between 60 and 80 °C. After this, the organoclay in suspension was concentrated in a centrifuge and washed 3–6 times with the initial solution, water and/or alcohol to remove the excess of organic modifier. For clay minerals modified with phosphonium 1 and phosphonium 2, the verification of the presence of halogen anion in the suspension was performed by adding 0.1 M silver nitrate in the filtrate. The agglomerates obtained were dried under vacuum at 60 °C for 24–48 h. The dried agglomerates were broken down to obtain powder materials that were sieved (mesh aperture: 0.074 mm) to posterior characterization.
Characterization techniques
X-ray diffraction (XRD)
The X-ray diffraction characterization of the clay minerals and organoclays was performed on a diffractometer Rigaku model Geigerflex equipped with CuKα radiation and Kβ-Ni filter. The measurements were taken in two angular ranges (2θ): from 1.7° to 75° and from 1.7° to 10°, and the scanning rates utilized were 1 and 0.25° min−1, respectively.
Chemistry analysis by X-ray fluorescence
The chemistry analysis by X-ray fluorescence of the unmodified clay minerals (Mt-1 Na+ and Mt-2 Na+) was performed on an equipment PW 1400 (Philips), using calibration curves with pattern NIST, BCS and IPT.
Infrared spectroscopy (FT-IR)
Infrared spectroscopy was performed on a spectrometer PerkinElmer, model Spectrum 1000. Spectrums of absorption were obtained from 400 to 4000 cm−1. Samples were produced in form of KBr pellets 2 mm of thick.
Thermogravimetry
The thermal stability of the unmodified and modified clay minerals was measured on a 2950 thermogravimetric analyser (TA Instruments), under nitrogen atmosphere, from 25 to 900 °C at heating rate of 20 °C min−1. The sample mass used in experiments was about 15 mg.
Results and discussion
Characterization of neat clay minerals
Table 1 provides the chemical analysis results for the two sodium clay minerals used in this work, and the XRD patterns of these clay minerals are plotted in Fig. 2. The XRD patterns indicate that the mineral composition of both clay minerals is very similar and that montmorillonite is the major constituting phase. The XRD patterns also indicate the presence of minor amount of minerals kaolinite and quartz. The content of SiO2 obtained by chemical analysis is due to the presence of the aluminosilicates montmorillonite and kaolinite and also due to the quartz phase in clay minerals. The aluminum (Al2O3) also comes from the montmorillonite and kaolinite phases. The presence of sodium and calcium mainly in montmorillonites clay minerals is probably due to exchange cations. The iron can be present in the clay minerals as impurity (e.g., oxide or hydroxide of iron) or as a substitute of aluminum atoms in the aluminum silicate sheet.
Characterization of organoclays
Infrared spectroscopy
FT-IR spectra of neat clay minerals (Mt-1 Na+ and Mt-2 Na+) and the organoclays are displayed in Figs. 3 and 4. The characteristic band at 3634 cm−1 corresponds to vibration of hydroxyl group linked to aluminum and magnesium [37]. Other important absorbance occurs at 1035 cm−1, due to stretching Si–O bonds of silicates present in the clay mineral and at 916 and 800 cm−1, due to vibrations of Al(Al)OH and Mg(Mg)OH in montmorillonite clay mineral, respectively. Peaks at 3400 and 1640 cm−1 correspond to the –OH stretching and bending vibration, respectively, of adsorbed H2O [37, 38]. The intensity of these H2O bands was reduced for organoclays in comparison with pristine montmorillonites, mainly for phosphoniums organoclays and at a lower extent for silane, showing that the intercalation process reduced the water content of organoclays. Further evidence of successful organic modification of Mt-1 and Mt-2 is given by the presence of peaks assigned to organic groups in FT-IR. Peaks found at 1466, 2850 and 2926 cm−1 can be assigned to stretching of C–C bonds, symmetric and asymmetric C–H vibrations, respectively, and are associated with the aliphatic chains of phosphonium, ammonium and silane organic modifiers [39, 40]. The spectra of the aromatic phosphonium 2 show additional peaks at 1440 and at 3066 cm−1, which can be attributed to stretching of C–C and C–H bonds, respectively, in aromatic rings [41, 42].
Thermogravimetric analysis (TG): thermal resistance of phosphonium organoclays
The thermogravimetric analysis curves (TG curves) for the pristine clay minerals and organoclays are shown in Fig. 5, and the derivative thermogravimetric curves (DTG), which are the first derivatives curves from TG curves, are shown in Fig. 6. By its turn, the thermal gravimetric parameters extracted from these curves are presented in Table 2. For temperature up to 130 °C, unmodified clay minerals Mt-1 and Mt-2 undergo a first mass loss of 8.3 and 7.7 mass%, respectively (ML130 in Table 2). This first mass loss is ascribed to water loss in montmorillonite from two sources: adsorbed water in pores (water molecules with large mobility) and water molecules from hydration shell of the exchangeable cations [17]. For phosphoniums and ammonium organoclays, the mass loss below 130 °C is much smaller than that of unmodified clay minerals, since the hydrated inorganic cations were exchanged by hydrophobic organic cations [43]. However, for silane organoclay, the values of ML130 are similar to the respective pristine montmorillonites, which shows that in this organoclay the hydrated inorganic cations are still present in interlayer space. This assumption is very reasonable, since it is expected that the silane molecules become adhered onto montmorillonite through adsorption and/or covalent bonding instead of cations exchanging. These values of water content on montmorillonites and organoclays given by TG analysis qualitatively corroborate the results found by FT-IR analysis. DTG curves show another peak of mass loss at 681 and 660 °C for unmodified montmorillonites Mt-1 and Mt-2, respectively, with a mass loss of about 6 mass%. These peaks of mass loss can be associated with dehydroxylation reactions of montmorillonite layers [43–45] and can be seen also in the DTG curves of the organoclays.
Besides the peaks in DTG curves associated with mass loss due to water elimination and dehydroxylation, there are additional peaks associated with thermal degradation of the organic molecules from ammonium, phosphoniums and silane compounds. Thermal stability for these organoclays was evaluated from some parameters extracted from TG and DTG curves, namely the onset temperature of organic degradation, maximum temperatures from peaks on DTG curves, temperature for 10 mass% of mass loss and mass loss at 275 °C. These parameters are summarized in Table 2 and demonstrate that the thermal stability of phosphonium organoclays is far superior to that of ammonium organoclay, what has also been shown in other works [44–46]. For instance, while the ammonium organoclay has an onset temperature for degradation of 156 °C, phosphonium 1 and phosphonium 2 organoclays have onset temperatures of 253 and 273 °C, respectively. It means an increase in the onset temperature of degradation by 97 and 117 °C for phosphonium 1 and phosphonium 2, respectively. Since 156 °C is in the temperature range used for processing of most thermoplastics, it is expected that ammonium organoclays undergo significant degradation when dispersed in such thermoplastics by melt compounding, what would not occur with phosphoniums organoclays. According to Xie et al. [44], the mechanisms of thermal degradation of aliphatic ammonium and phosphonium organoclays are similar, i.e., β-elimination (Hoffman process) and nucleophilic substitution (of N or P atoms). The higher thermal stability of phosphoniums as compared to ammoniums counterparts is due to the differences between the atomic characteristics of phosphorous and nitrogen, what changes the onset temperature of each one of these mechanisms. For example, nitrogen has higher electronegativity than phosphorous, what promotes a higher acidity of β-protons in alkyl ammonium than in alkyl phosphonium compounds [47]. Thus, in the presence of base, these β-protons in ammonium organocations undergo facile Hoffman elimination, unlike the β-protons in phosphonium [44, 48, 49].
Regarding the effect of the type of phosphonium organic modifier, the onset temperatures of degradation and the peak degradation temperatures from DTG curves are higher for phosphonium 2 than phosphonium 1 whatever the montmorillonite of the organoclay (Mt-1 or Mt-2). For Mt-1 phosphonium organoclays, the onset temperature of degradation of phosphonium 2 is 20 °C larger than phosphonium 1 (273 vs. 253 °C), while for Mt-2 organoclays this difference is 9 °C (247 vs. 238 °C). These results indicate that phosphonium with aryl groups presents higher thermal stability than the phosphonium with alkyl groups. This probably occurs because the phenyl groups of phosphonium 2 promote a steric hindrance around the phosphorous atom, protecting this atom from the attack of ions and free radicals. It is also possible that interactions between phosphorous atom and phenyl groups result in the delocalizations of the positive charge of phosphonium cation, what can reduce the acidity of β-protons of the alkyl chain and therefore reduce the possibility of β-elimination reactions [44].
In order to analyze the effect of montmorillonite type on the thermal stability of phosphonium organoclays, TG thermal parameters from Table 2 of Mt-1 phosphonium organoclays are compared with that of Mt-2 organoclays with the same organic modifier. For phosphonium 1 organoclays, Mt-1 organoclay has a larger onset temperature of degradation (15 °C larger) and larger temperature of peak of mass loss (45 °C larger) as compared with Mt-2 organoclay with the same phosphonium 1. For phosphonium 2 organoclays, Mt-1 organoclay has an onset temperature of degradation 26 °C larger than that of Mt-2 organoclay with the same phosphonium 2, even though the temperature of peak of mass loss is similar (about 530 °C). In addition, the behavior of degradation peaks of DTG curves (Fig. 6a, b) changes significantly from Mt-1 to Mt-2 organoclays for the same phosphonium molecule. For instance, the first organic degradation peak of phosphonium 2/Mt-2 (Fig. 6b) is significantly larger than the peak of phosphonium 2/Mt-1 (Fig. 6a).
Xie et al. [44, 48] have argued that thermal degradation behavior of surfactants in organoclays is quite different from the neat surfactants due to catalytic activity of aluminosilicate platelets and the nanoscopic confinement of the organic molecules inside these platelets. The catalytic activity depends on the concentration of Bronsted and Lewis acid centers originated from hydroxyl groups and partially coordinated metal atoms, and this activity is expected to decrease the onset temperature of degradation of surfactants. By its turn, the nanoscopic morphology of the surfactant inside the platelets is expected to dictate the behavior of the multiple degradation peaks in DTG curves. Thus, the observed lower thermal stability of Mt-2 phosphoniums organoclays compared with Mt-1 suggests that Mt-2 aluminosilicate has a larger catalytic activity than Mt-1. Moreover, the very distinct behavior of DTG curves of Mt-1 and Mt-2 phosphoniums organoclays suggests that these two organoclays have very different interlayer confinement and arrangement of phosphoniums molecules. This speculation is corroborated by the results of the analysis of organic content and interlayer distance, which are discussed further ahead.
Thermogravimetric analysis (TG): thermal resistance of silane organoclay
Silane organoclays present an onset temperature of degradation of 254 and 252 °C for organoclays with Mt-1 and Mt-2, respectively. Moreover, the peak degradation temperatures are 412 and 403 °C for silane/Mt-1 and silane/Mt-2, respectively. These values suggest that silane/Mt-1 organoclay has a higher thermal stability than ammonium/Mt-1 organoclay. On the other hand, the values of peak degradation temperatures from Table 2 indicate that the thermal stability of silane organoclays is lower than that of phosphoniums organoclays. Another feature of silane organoclays is the minor influence of the type of aluminosilicate (Mt-1 or Mt-2) on its thermal stability as compared with phosphonium organoclays. In fact, the values of onset temperature of degradation and temperatures of peaks of mass loss are similar to silane organoclays with Mt-1 and Mt-2. Moreover, the degradation curves of silane organoclay present just a single degradation peak (about 400 °C) in DTG curves. This differs from the other organoclays, which have more than one degradation peak assigned to organic degradation. As discussed above, multiple steps degradation has been associated with the variety of environment of organic molecules in the interlayers galleries [49]. The absence of staged degradation in silane organoclays suggests that the kind of interaction of the organic silane molecules with the inorganic montmorillonite platelets is different from the interactions expected for the ammonium and phosphoniums molecules. While for ammonium and phosphonium molecules it is expected electrostatic interaction of nitrogen or phosphorous atoms with the inorganic platelets, for silane molecules it is expected the following interactions: physically adsorbed silane molecules onto montmorillonite surface or in the interlayers gallery and covalent-bonded silane molecules on the edges of the clay mineral platelets or on the interlayer surface of these platelets [49, 50].
Thermogravimetric analysis (TG): organic content
Besides evaluating the thermal resistance of organoclays, TG analysis is also useful to estimate the organic content of these compounds. The organic contents for each organoclay were calculated from the respective TG curves based on the assumption that the loss of water, organic degradation and dehydroxylation process can be assigned to discrete steps in these curves and that the respective DTG peaks for each process do not overlap each other [51]. Thus, the organic content in each TG curve was determined from the mass content values between the onset temperatures of organic loss and the dehydroxylation. By its turn, these temperatures were assumed as the onset temperatures of the DTG peaks associated with these two mass loss events. The values of organic contents (OC) calculated through this method are shown in Table 2.
From the organic contents values, the values of CEC coverage were calculated as the ratio of molar concentration of the surfactants to the CEC values and are also shown in Table 2. The CEC coverage values for phosphonium organoclays are below 1, suggesting that exchange reaction between the sodium and phosphonium cations was incomplete, even though the process of organic modification of clay minerals was performed with a concentration of organic modifier relative to clay minerals of about 1.5 times the CEC of the clay mineral. One possible reason for this incomplete exchange is the large size of phosphoniums surfactants, what would hamper the diffusion of these organic in the gallery of the montmorillonites. Another possible reason is related to solubility issues during the organic intercalation process, since phosphoniums surfactants are not soluble in water at room temperature and thus it is necessary increase water temperature or adds ethanol in order to promote solubilization. Therefore, it is possible that, in the step of mixing the phosphonium solutions in the montmorillonite suspension, some precipitation of phosphonium or aggregation of montmorillonite may have happened.
The CEC coverage is also influenced by the montmorillonite type, since the CEC coverage value for Mt-1/phosphonium is larger than Mt-2/phosphonium, comparing with the same phosphonium modifier. It is likely that the exchange reaction between the sodium and phosphonium cations in Mt-2 clay mineral was incomplete because of the higher charge density of platelets in this clay mineral, as can be deduced from its higher CEC. This increased charge is expected to promote a stronger electrostatic attraction between the clay mineral platelets and the interlayer cations, hampering the entrance of bulk phosphonium cations into the clay mineral galleries. It is also possible that the higher CEC of Mt-2 pristine montmorillonite precluded a complete exfoliation of platelets in water during the intercalation process, mainly when alcoholic solution of phosphonium was added in Mt-2 suspension. This difference in CEC between phosphonium organoclays with Mt-1 and Mt-2 is another evidence that these organoclays have distinct interlayer confinement and arrangement of phosphonium molecules, what corroborates the above discussion about the effect of montmorillonite type on thermal degradation behavior of phosphonium organoclays.
X-ray diffraction analysis
The intercalation of the molecules of organic modifiers between layers of the clay minerals was accompanied by XRD, through the analysis of the basal spacing d 001 of the organoclays. XRD patterns of the organically modified Mt-1 and Mt-2 clay minerals are plotted in Figs. 7 and 8, respectively. For the sake of comparison, besides the XRD patterns of clay minerals modified with phosphonium and silane, diffractogram of Mt-1 30B which contains conventional ammonium quaternary salts on the structure is also plotted in Fig. 7. Table 3 summarizes the interplanar distances d 001 of organoclays calculated from the diffraction angles of the correspondent 001 reflections in Figs. 7 and 8. All the organic modifiers promoted an expansion of the interlayer spacing between the clay mineral platelets, since all the XRD patterns of modified clay minerals showed 001 reflections displacement to the left compared to unmodified clay minerals. Regarding the effect of the type of organic modifier on the basal spacing, from the results of Table 3, we can rank the modifiers in the following order in terms of capacity of increasing the basal spacing: phosphonium 1 > phosphonium 2 ~ ammonium surfactant > silane. For phosphonium surfactants, Patel et al. [41] have calculated the maximum length of various structures of phosphoniums surfactants. From these surfactants, the structures of tributyl tetradecyl phosphonium bromide (P3) and propyl triphenyl phosphonium bromide (P7) are similar to structures of phosphonium 1 and phosphonium 2, respectively, and it was found a length of 1.0 nm for P3 and 0.4 nm for P7. Even though the structures of phosphonium 1 and phosphonium 2 are not identical to that from Patel’s work, it serves as a qualitative comparison between the sizes of these two surfactants and therefore the length of phosphonium 1 can be considered to be larger than that of phosphonium 2, which is coherent with the larger basal spacings of phosphonium 1 organoclays. This observation is in accordance with the results of some works which have shown that the longer the length of aliphatic chains of the organic modifier the larger the layer spacing [39, 52]. Moreover, from the values of basal spacing of phosphonium organoclays, we can speculate about the most probable configuration of these surfactants in the interlayer gallery. For organoclays of Mt-1 and Mt-2 with phosphonium 1, the d 001 values are 2.33 and 2.10 nm, respectively. These values are consistent with a pseudo-trilayer and a bilayer arrangement of the aliphatic chains, respectively. In another hand, for organoclays with phosphonium 2, the d 001 values is 1.84 nm for Mt-1 and 1.77 nm for Mt-2, which suggests a bilayer arrangement of the phosphonium 2 chains [45, 53–56].
The incorporation of silane on montmorillonite promoted a smaller increasing of interplanar distance, as compared with that promoted by phosphonium molecules, what suggests that there is a low concentration of silane molecules intercalated into the gallery of montmorillonite. Some studies have shown that when water or water/ethanol is used as the solvent on the silylation process (as in the present work) the silane molecules can diffuse into the clay mineral platelets, what could lead to a reasonable intercalation degree of silane molecules [49, 50]. However, depending on the intercalation conditions, the solution water/ethanol can promote a premature hydrolysis and condensation of silane molecules before the intercalation of initial molecules takes place. In this case, these reactions can generate polymers with large sizes, which are more difficult to be intercalated between the clay mineral galleries, leading to a decrease in intercalation [57]. This is a reasonable explanation for the small increase in interplanar spacing after silylation in this work, mainly because the silane used here is bifunctional, and therefore, premature hydrolysis and condensation could generate large cross-linked siloxane polymers.
The small increase in interplanar spacing after the silylation can also be alternatively explained by take in account that montmorillonite sheets have silanol groups on its edges which can react with the silane molecules. It has been estimated that the negative charge assigned to these silanol groups is about 15 mass% of the total clay mineral charge [35, 36, 58]. By considering the model proposed by Herrera et al. [32] which argue that mostly of the silane molecules react with silanol groups present at the montmorillonite sheet edges, it is expected that the silane molecules attached by this way into one clay mineral sheet edge can further react with other silane molecules from neighbor sheets, since the silane molecules are bifunctional. Therefore, a cross-linked network of siloxane polymer can be generated around the clay mineral edges sheets, what can push these sheets apart and slightly increase the d 001 value.
Besides the influence of organic modifier, the montmorillonite type also influences the basal spacing of organoclays, since the basal spacing of Mt-1 organoclays is slightly larger than that of Mt-2 ones, i.e., the larger the CEC of the clay mineral the smaller the increment in basal spacing of organoclay relative to pristine montmorillonite. One could expect an opposite trend, i.e., an increase in basal spacing with the increase in CEC, since a clay mineral with higher CEC could accommodate more organic modifier into its gallery if all the sodium cations were replaced by the organic cations [59–63]. Such an opposite trend in the present work can be due to an incomplete exchange reaction between the sodium and phosphonium cations in Mt-2 clay mineral, what is likely to have occurred, as was seen by the CEC coverage results shown above. In fact, a comparison between Tables 2 and 3 reveals that the lower CEC coverage of phosphonium/Mt-2 organoclays is correlated with lower basal distance of these organoclays, as compared with phosphonium/Mt-1 organoclays. As has been shown elsewhere [54, 64–68], besides the CEC, the basal spacing also depends on the organic loading of the clay mineral relative to its CEC.
Conclusions
In this study, organoclays containing ammonium, phosphonium and silane were prepared and characterized, in order to evaluate its suitability for utilization in polymer nanocomposites prepared by mixing these organoclays with polymers in the melt state. The selection of the organoclay for this application should consider not only the thermal stability but also the interplanar distance of the organoclay, since larger galleries can facilitate the intercalation of polymer molecules into organoclays during the processing in the melt state.
Overall, the thermal stability of the organoclays studied decreases in the order: phosphoniums > silane > ammonium. By its turn, the lamellar spacing between aluminosilicate platelets decreases in the order: phosphoniums > ammonium > silane. This indicates that phosphoniums would be the most preferable for mixing in polymer melts, due to its highest thermal stability and lamellar spacing. Between silane and ammonium, silane would be a better choice regarding the thermal stability, but one would have to evaluate whether the low interplanar distance of this organoclay would preclude the intercalation of polymeric chains during the preparation of nanocomposites by melt compounding. Between phosphoniums organoclays studied, phosphonium 2 (containing phenyl groups) presents higher thermal resistance but lower lamellar spacing than phosphonium 1. Thus, phosphonium 2 would be a better choice than phosphonium 1 for use in nanocomposites if its lower lamellar spacing does not hinder the polymeric intercalation process. Finally, it was also found evidences that the montmorillonite source have a significant influence on thermal stability, behavior of thermal degradation and lamellar spacing of the phosphonium organoclays, at least for the method of preparation of the organoclays used in this work.
References
Grim RE. Clay mineralogy. New York: McGraw-Hill; 1968.
Alther GR. Organically modified clay removes oil from water. Waste Manag. 1995;15:623–8.
Xu SH, Sheng G, Boyd SA. Use of organoclays in pollution abatement. Adv Agron. 1997;59:25–62.
Beall GW. The use of organo-clays in water treatment. Appl Clay Sci. 2003;24:11–20.
Churchman GJ, Gates WP, Theng BKG, Yuan G. Clays and clay minerals for pollution control. In: Bergaya F, Theng BKG, Lagaly G, editors. Handbook of clay science. Amsterdam: Elsevier; 2006. p. 625–75.
Solomon DH, Hawthorne DG. Chemistry of pigments and fillers. New York: Wiley; 1983.
Pinnavaia TJ, Beall GW. Polymer–clay nanocomposites. Chichester: Wiley; 2000.
Usuki A, Kojima Y, Kawasumi M, Okada A, Fukushima Y, Kurauchi T, Kamigaito O. Synthesis of nylon 6-clay hybrid. J Mater Res. 1993;8:1179–84.
Sinha Ray S, Okamoto M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci. 2003;28:1539–641.
Paul DR, Robeson LM. Polymer nanotechnology: nanocomposites. Polymer. 2008;49:3187–204.
Souza MA, Larocca NM, Araujo EM, Pessan LA. Preparation and characterization of nanocomposites of polyamide 6/Brazilian clay with different organic modifiers. Mater Sci Forum. 2008;570:18–23.
Martins CG, Larocca NM, Paul DR, Pessan LA. Nanocomposites formed from polypropylene/EVA blends. Polymer. 2009;50:1743–54.
Oliveira AD, Larocca NM, Paul DR, Pessan LA. Effects of mixing protocol on the performance of nanocomposites based on polyamide 6/acrylonitrile-butadiene-styrene blends. Polym Eng Sci. 2012;52:1909–19.
Vaia RA, Giannelis EP. Lattice model of polymer melt intercalation in organically-modified layered silicates. Macromolecules. 1997;30:7990–9.
Vaia RA, Giannelis EP. Polymer melt intercalation in organically-modified layered silicates: model predictions and experiment. Macromolecules. 1997;30:8000–9.
Manias E, Chen H, Krishnamoorti R, Genzer J, Kramer EJ, Giannelis EP. Intercalation kinetics of long polymers in 2 nm confinements. Macromolecules. 2000;33:7955–66.
Xie W, Gao Z, Liu K, Pan WP, Vaia R, Hunter D, Singh A. Thermal characterization of organically modified montmorillonite. Thermochim Acta. 2001;367–368:339–50.
Leszezynska A, Njuguna J, Pielichowski K, Banerjee JR. Polymer/montmorillonite nanocomposites with improved thermal properties. Part I. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim Acta. 2007;453:75–96.
Yoon PJ, Hunter DL, Paul DR. Polycarbonate nanocomposites: part 2. Degradation and color formation. Polymer. 2003;44:5341–54.
Fornes TD, Yoon PJ, Paul DR. Polymer matrix degradation and color formation in melt processed nylon 6/clay nanocomposites. Polymer. 2003;44:7545–56.
Gilman JW, Awad WH, Davis RD, Shields J, Harris RH Jr, Davis C, Morgan AB, Sutto TE, Callahan J, Trulove PC, DeLong HC. Polymer/layered silicate nanocomposites from thermally stable trialkylimidazolium-treated montmorillonite. Chem Mater. 2002;14:3776–85.
Há JU, Xanthos M. Functionalization of nanoclays with ionic liquids for polypropylene composites. Polym Compos. 2009;30:534–42.
Livi S, Duchet-Rumeau J, Pham TN, Gérard JF. A comparative study on different ionic liquids used as surfactants: effect on thermal and mechanical properties of high-density polyethylene nanocomposites. J Colloid Interfaces Sci. 2010;349:424–33.
Ganguly S, Dana K, Mukhopadhyay TK, Ghatak S. Simultaneous intercalation of two quaternary phosphonium salts into montmorillonite. Clays Clay Miner. 2011;59:13–20.
Ganguly S, Dana K, Mukhopadhyay TK, Ghatak S. Thermal degradation of alkyl triphenyl phosphonium intercalated montmorillonites—an isothermal kinetic study. J Therm Anal Calorim. 2011;105:199–209.
Livi S, Duchet-Rumeau J, Gérard J. Supercritical CO2—ionic liquid mixtures for modification of organoclays. J Colloid Interfaces Sci. 2011;353:225–30.
Yan Z, Meng D, Huang Y, Hou Z, Wu X, Wang Y, Du X, Xie H. Modification of kaolinite with alkylimidazolium salts. J Therm Anal Calorim. 2014;118:133–40.
Isoda K, Kuroda K. Interlamellar grafting of γ-methacryloxypropylsilyl groups on magadiite and copolymerization with methyl methacrylate. Chem Mater. 2000;12:1702–7.
Shimojima A, Mochizuki D, Kuroda K. Synthesis of silylated derivatives of a layered polysilicate kanemite with mono-, di-, and trichloro(alkyl)silanes. Chem Mater. 2001;13:3603–9.
Park KW, Jeong SY, Kwon OY. Interlamellar silylation of H-kenyaite with 3-aminopropyltriethoxysilane. Appl Clay Sci. 2004;27:21–7.
Park M, Shim IK, Jung EY, Choy JH. Modification of external surface of laponite by silane grafting. J Phys Chem Solids. 2004;65:499–501.
Herrera NN, Letoffe J, Putaux J, David L, Bourgeat-lami E, Lyon CB, Cedex V. Aqueous dispersions of silane-functionalized laponite clay platelets. a first step toward the elaboration of water-based polymer/clay nanocomposites. Langmuir. 2004;20:1564–71.
Su L, Tao Q, He H, Zhu J, Yuan P, Zhu R. Silylation of montmorillonite surfaces: dependence on solvent nature. J Colloid Interfaces Sci. 2013;391:16–20.
Bruce AN, Lieber D, Hua I, Howarter JA. Rational interface design of epoxy-organoclay nanocomposites: role of structure–property relationship for silane modifiers. J Colloid Interfaces Sci. 2014;419:73–8.
Fletcher P, Sposito G. The chemical modeling of clay electrolyte interactions for montmorillonite. Clay Miner. 1989;24:375–91.
Morris HD, Bank S, Ellis PD. 27AI NMR spectroscopy. J Phys Chem. 1990;3:3121–9.
Frost RL, Kloprogge JT. Vibrational spectroscopy of ferruginous smectite and nontronite. Spectrochim Acta Part A. 2000;56:2177–89.
Madejova J. FTIR techniques in clay mineral studies. Vib Spectrosc. 2003;31:1–10.
Vaia RA, Teukolsky RK, Giannelis EP. Interlayer structure and molecular environment of alkylammonium layered silicates. Chem Mater. 1994;6:1017–22.
Xi YF, Ding Z, He HP, Frost RL. Infrared spectroscopy of organoclays synthesized with the surfactant octadecyltrimethylammonium bromide. Spectrochimica Acta Part A. 2005;61:515–25.
Pate HA, Somani RS, Bajaj HC, Jasra RV. Preparation and characterization of phosphonium montmorillonite with enhanced thermal stability. Appl Clay Sci. 2007;35:194–200.
Ganguly S, Dana K, Parya TK, Mukhopadhyay TK, Ghatak S. Organic-inorganic hybrids prepared from alkyl phosphonium salts intercalated montmorillonites. Ceramics Silikáty. 2012;56:306–13.
He H, Ding Z, Zhu J, Yuan P, Xi Y, Yang D, Frost RL. Thermal characterization of surfactant-modified montmorillonites. Clays Clay Miner. 2005;53:287–93.
Xie W, Xie R, Pan W, Hunter D, Koene B, Tan L, Vaia R. Thermal stability of quaternary phosphonium modified montmorillonites. Chem Mater. 2002;15:4837–45.
Hedley CB, Yuan G, Theng BKG. Thermal analysis of montmorillonites modified with quaternary phosphonium and ammonium surfactants. Appl Clay Sci. 2007;35:180–8.
Leite IF, Soares AP, Carvalho LH, Raposo CMO, Malta OML, Silva SML. Characterization of pristine and purified organobentonites. J Therm Anal Calorim. 2010;100:563–9.
Abdallah DJ, Weiss RG. The influence of the cationic center, anion, and chain length of tetra-n-alkylammonium and -phosphonium salt gelators on the properties of their thermally reversible organogels. Chem Mater. 2000;12:406–13.
Xie W, Gao Z, Pan WP, Hunter D, Singh A, Vaia R. Thermal degradation chemistry of alkyl quaternary ammonium Montmorillonite. Chem Mater. 2001;13:2979–90.
He H, Duchet J, Galy J, Gerard J. Grafting of swelling clay materials with 3-aminopropyltriethoxysilane. J Colloid Interfaces Sci. 2005;288:171–6.
Shanmugharaj AM, Yop K, Hun S. Influence of dispersing medium on grafting of aminopropyltriethoxysilane in swelling clay materials. J Colloid Interfaces Sci. 2006;298:854–9.
Ek S, Root A, Peussa M, Niinistö L. Determination of the hydroxyl group content in Silica by thermogravimetry and a comparison with H-1 MAS NMR results. Thermochim Acta. 2001;379:201–12.
He H, Ma Y, Zhu J, Yuan P, Qing Y. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl Clay Sci. 2010;48:67–72.
Torok B, Bartok M, Dekany I. The structure of chiral phenyl-ethylammonium montmorillonites in ethanol–toluene mixtures. Colloid Polym Sci. 1999;277:340–6.
Imai Y, Nishimura S, Inukai Y, Tateyama H. Differences in quasicrystals of smectite-cationic surfactant complexes due to head group structure. Clays Clay Miner. 2003;51:162–7.
Lagaly G, Ogawa M, Dékány I. Clay mineral-organointeractions. In: Bergaya F, Theng BKG, Lagaly G, editors. Handbook of clay science. Amsterdam: Elsevier; 2006. p. 309–77.
Mirau PA, Serres JL, Jacobs D, Garrett PH, Vaia RA. Structure and dynamics of surfactant interfaces in organically modified clays. J Phys Chem B. 2008;112:10544–51.
Shen W, He H, Zhu J, Yuan P, Frost RL. Grafting of montmorillonite with different functional silanes via two different reaction systems. J Colloid Interfaces Sci. 2007;313:268–73.
Tournassat C, Neaman A, Villéras F, Bosbach D, Charlet L. Nanomorphology of montmorillonite particles: estimation of the clay edge sorption site density by low-pressure gas adsorption and AFM observations. Am Mineral. 2003;88:1989–95.
Lan T, Kaviratna PD, Pinnavaia TJ. Mechanism of clay tactoid exfoliation in epoxy–clay nanocomposites. Chem Mater. 1995;7:2144–50.
Hackett E, Manias E, Giannelis EP. Molecular dynamics simulations of organically modified layered silicates. J Chem Phys. 1998;108:7410–74.
Fornes TD, Hunter DL, Paul DR. Effect of sodium montmorillonite source on nylon 6/clay nanocomposites. Polymer. 2004;45:2321–31.
Ganguly S, Dana K, Ghatak S. Thermogravimetric study of n-alkylammonium-intercalated montmorillonites of different cation exchange capacity. J Therm Anal Calorim. 2010;100:71–8.
Mauroy H, Plivelic TS, Hansen EL, Fossum JO, Helgesen G, Knudsen KD. Effect of clay surface charge on the emerging properties of polystyrene–organoclay nanocomposites. J Phys Chem C. 2013;117:19656–63.
Paul DR, Zeng QH, Yu AB, Lu GQ. The interlayer swelling and molecular packing in organoclays. J Colloid Interfaces Sci. 2005;292:462–8.
Li Z, Jhang WT. Interlayer conformations of intercalated dodecyltrimethylammonium in rectorite as determined by FTIR, XRD, and TG analysis. Clays Clay Miner. 2009;57:194–204.
Fraser KJ, MacFarlane DR. Phosphonium-based ionic liquids: an overview. Austr J Chem. 2009;62:309–21.
Park Y, Ayoko GA, Kristof J, Horváth E, Frost RL. A thermoanalytical assessment of an organoclay. J Therm Anal Calorim. 2012;107:1137–42.
He H, Ma L, Zhu J, Frost RL, Theng BKG, Bergaya F. Applied clay science synthesis of organoclays: a critical review and some unresolved issues. Appl Clay Sci. 2014;100:22–8.
Acknowledgements
The authors would like to thank the Brazilian National Research Council for Science and Technology (CNPq) for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souza, M.A., Larocca, N.M. & Pessan, L.A. Highly thermal stable organoclays of ionic liquids and silane organic modifiers and effect of montmorillonite source. J Therm Anal Calorim 126, 499–509 (2016). https://doi.org/10.1007/s10973-016-5501-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5501-z