Abstract
High resolution thermogravimetric analysis (TG) has attracted much attention in the synthesis of organoclays and its applications. In this study, organoclays were synthesised through ion exchange of a single cationic surfactant for sodium ions, and characterised by methods including X-ray diffraction (XRD) and TG. The changes of surface properties in montmorillonite (MMT) and organoclays intercalated with surfactant were determined using XRD through the changes in the basal spacing. The TG was applied in this study to investigate more information of the configuration and structural changes in the organoclays with thermal decomposition. There are four different decompositions steps in differential thermogravimetric curves. The obtained TG steps are relevant to the arrangement of the surfactant molecules intercalated in MMT and the thermal analysis indicates the thermal stability of surfactant modified clays. This investigation provides new insights into the properties of organoclays and is important in the synthesis and processing of organoclays for environmental applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The abundant clay minerals in nature are widely used in a various range of industries such as nano composites, catalysts, photochemical reaction reagents and adsorbents [1]. Among other applications, clays have extensively been used as adsorbents in environmental applications. However, the clay mineral surface is hydrophilic in nature and this clay surface characteristic makes the natural clays ineffective sorbents for the removal of organic compounds. Such a difficulty has been overcome by introducing cationic surfactant molecules into the interlamellar space, and the properties of clay minerals are enhanced as organoclays. In recent years, organoclays have been used of oil-spill clean-up operations [2–4], and also proposed for the removal of organic contaminants [5–8]. The most commonly used clay for the preparation of organoclays is montmorillonite (MMT) due to its high cation exchange capacity (CEC), swelling properties, high surface areas and consequential strong adsorption/absorption [9]. The typical 2:1 layered MMT, which consists of two siloxane tetrahedral sheets sandwiching an aluminium octahedral sheet belongs to the swelling group of clays [10]. The swelling clays tend to swell and hydrate upon exposure to water. Because of the isomorphous substitution within the layers (e.g. the replacement of Mg2+ or Zn2+ for Al3+ in the octahedral layer, and Al3+ for Si4+ in the tetrahedral sheets), the negatively charged clay layer surfaces are counterbalanced by exchangeable cations such as Na+ or Ca2+ in the interlayer space.
The intercalation of organic cations such as quaternary ammonium cations between clay layers changes the surface properties from highly hydrophilic to increasingly hydrophobic/lipophilic [11]. As followed by the modification of clays with cationic surfactants, basal spacing in the layer increases and new sorption sites of clays are exposed. Such surfactant surface property changes are highly important for the application of organoclays. In particular, the organoclays are seen to be considerably more effective than untreated clay minerals for the removal of organic contaminants [7, 12]. Furthermore, organoclays-based nanocomposites exhibit a remarkable improvement in properties when compared with untreated polymer or conventional micro- and macrocomposites [10]. The improvements include increased strength and heat resistance, decreased gas permeability and flammability and increased biodegradability. Hence, the industrial applications and improvements are heavily dependent on the structure and properties of organoclays, which requires to be identified [13].
The application of thermal analysis to the study of clays, intercalated kaolinites [14–18], layered double hydroxides [19–22] and nanomaterials [23, 24] is of great significance and is essential for the advancement of knowledge and understanding of clay minerals and their intercalates. In this study, hexadecyltrimethylammonium bromide (HDTMA) is chosen as an example of a long alkyl cationic surfactant for the preparation of organoclays, and the effect of the long alkyl surfactant with different loadings may increase the utilisation of modified clays. This study was also undertaken to investigate the structure of organoclays using X-ray diffraction and the interlayer configuration of intercalated surfactant in MMT was elucidated. Moreover, thermogravimetric analysis (TG) was used to investigate the microenvironment and packing arrangement of organic surfactant within the organoclays. The results obtained in this investigation will offer new insights into the structure and properties of organoclays, and will further lead to potential applications of organoclays in industry applications.
Experimental
Materials
The pure MMT used in this study was supplied by Sigma-Aldrich as source clay SWy-2-Na-MMT (Wyoming) and this was used without further purification. The CEC of this MMT is 76.4 meq/100 g. The surfactant selected for the organoclay complexes in this study is hexadecyltrimethylammonium bromide (denoted as HDTMA, C19H42BrN, FW: 364.46) from Sigma-Aldrich, and used without any further purification.
Synthesis of organoclays
The synthesis of organoclay complexes was undertaken by the following procedure: 4 g of MMT was initially dispersed in 400 mL of deionised water with a Heidolph magnetic stirrer for about 30 min. A predissolved stoichiometric amount of surfactant, which was dissolved in 100 ml of deionised water, was stirred for a further 30 min. The dissolved surfactant was slowly added to the clay suspension at room temperature (about 28–30 °C). The CEC of the MMT is 76.4 meq/100 g, which represents a measure of the loading of the clay with the cationic surfactant. For instance, 1.0 CEC is applied when 76.4 meq/100 g is intercalated into the MMT. During the synthesis, a range of surfactant concentration in terms of the CEC value from 0.25 CEC through 2.0 CEC was prepared and labelled as 0.25 CEC-HDTMA, 0.50 CEC-HDTMA, 1.0 CEC-HDTMA, 1.5 CEC-HDTMA and 2.0 CEC-HDTMA. The mixtures were stirred for 3 h at room temperature using a Branson Ultrasonic model 250 sonifier with an output of 40 mW. All organoclay products were washed free of bromide anions as determined by the use of AgNO3, dried at room temperature, and dried further in an oven (at 65 °C) for 12 h. The dried organoclays were ground in an agate mortar, and stored in vacuum desiccators for a week.
Characterisation methods
X-ray diffraction
The pure MMT and organoclays were present in stainless sample holders. The powdered X-ray diffraction (XRD) patterns recorded using CuK radiation (λ = 1.5418 Å) on a Philips PA Nalytical X’Pert PRO diffractometer operating at 40 kV and 40 mA with 0.5° divergence slit, 1° anti-scatter slit, between 3° and 70° (2θ) at a step size of 0.0167°.
Thermogravimetric analysis
Thermogravimetric analysis of the modified clays were obtained by using Netzsch TG 209 type Thermobalance at ramp 10 K/min from room temperature to 900 °C in high purity flowing argon (80%) with 20% of oxygen. Approximately 7–11 mg of finely dried ground samples was heated in an open ceramic crucible.
Results and discussions
X-ray diffraction
X-ray diffraction is one of the most important techniques to determine the structural geometry, texture and also identify impurities in layer silicates which are present in clays. In general, XRD can offer basal spacing information on the organoclays used, which can also provide the molecular structure configuration of surfactants intercalated in clay layers. The XRD patterns of MMT and organoclays prepared at different surfactant loadings are shown in Fig. 1. The sodium exchanged MMT has a d-spacing of 1.25 nm which has an expansion of 1.40 nm when ion exchanged with 0.25 CEC surfactant concentration level. The expansion at the 0.5 CEC surfactant level results in the expansion of the organoclays to 1.44 nm. Upon increasing the surfactant loading to 1.0 CEC surfactant concentration, d(001) spacing of 1.82 nm was observed. Organoclays with 1.5 CEC surfactant concentration level have the expansion peak at 1.80 nm, which has a slight smaller peak than that of organoclays with 1.0 CEC surfactant concentration level. The slight decreasing value may be caused by insufficient cationic surfactant added to displace all of the water from the interlayer space. The increasing basal spacing of untreated MMT and organoclays is clearly seen in Fig. 2.
The basal spacing at 2.0 CEC surfactant concentration level reaches of 2.04 nm. Depending on the concentration of surfactant, HDTMA, the expansion of the layers in each step has been appeared. Based on the result, the configuration structure of molecules between clay unit layers suggested. In 0.25 CEC-HDTMA and 0.50 CEC-HDTMA, the d values are 1.40 and 1.44 nm, respectively, and it implies a lateral monolayer arrangement in the interlayer space of MMT. For 1.0 CEC-HDTMA, it shows a basal spacing at 1.82 nm, which reflecting a lateral bilayer arrangement. At 2.0 CEC-HDTMA, the d basal spacing reaches up to 2.0 nm which reflects a pseudotrimolecular layer arrangement. This demonstrates the property variation among MMT layers. A model is proposed in which up to 0.5 CEC a surfactant monolayer is formed between the montmorillonitic clay layers, up to 1.0 CEC a lateral bilayer arrangement is formed with excess surfactant adsorbed onto the clay surface, a pseudotrimolecular layer is formed at 2.0 CEC with excess surfactant adsorbed onto the clay surface [25]. The loaded surfactant in the interlayer space of MMT has been further investigated by TG.
Thermogravimetric analysis
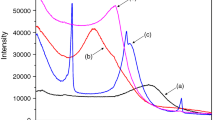
Thermal stability of organoclays and packing arrangement of organic surfactant within the organoclays at an elevated temperature can be determined by thermogravimetric analysis (TG). Figures 3 4, 5, 6, 7 show the derivative thermogravimetric analyses (DTG) of the organoclays intercalated with surfactant, HDTMA. The results of the analyses of the mass loss and temperature of the mass loss are reported in Table 1. The result of untreated MMT without intercalation with the surfactant is noted that there are three mass loss steps at between ambient and 100 °C, second at 135.5 °C, and at 636.3 °C [25]. From the result, the MMT is relatively stable and it begins to lose structural hydroxyl OH units at 636 °C but will maintain the layer structure up to ~900 °C [26].
From the Figures and Table 1, there are several mass loss steps observed. The first two steps of mass loss involve dehydration and/or dehydroxylation from room temperature and before 100 °C. The two steps are attributed to the loss of adsorbed water, and water molecules around metal cations such as Na+ and Ca2+ on exchangeable sites in MMT. The first mass loss for dehydration/dehydroxylation of adsorbed water varies from 2.14 to 7.17%. The experimental mass loss during the second hydration and hydroxylation of adsorption water appears only for untreated MMT. The first DTG peak for the desorption of water from the clay is highly intense in 0.25 CEC-HDTMA, and this mass loss peak becomes less with the increase of surfactant concentration loading. The third mass loss step is considered as the removal of surfactant by comparing the peak of pure surfactant. It is noted that the temperature of de-surfactant from organoclays is higher than the boiling temperature of pure surfactant. With the increase of surfactant concentration, the temperature of the third mass loss peak is gradually decreased. From the results, it is concluded that the loading of surfactant affects the de-surfactant mass loss and the temperature is decreased. When the concentration of the organoclay is relatively low (for 0.25 CEC and 0. 50 CEC), the organic cations exchanged with Na+ ions mainly adhere to surface sites via electrostatic interactions. With the increase of surfactant concentration loading to 1.0 CEC, some surfactant molecules tend to attach to the clay surface, which results in the appearance of the peak at 278 °C. When the surfactant loading has exceed over the CEC of clay for 1.5 CEC-HDTMA and 2.0 CEC-HDTMA, the surfactant molecules strongly adhere to clay surface by van der Waals forces, and this causes the peak decreased at 265 and 263 °C, respectively. In addition, the intensity of peak is increased with the increase of surfactant loading. During the de-surfactant procedure, the experimental mass loss increases from 2.91% (0.25 CEC-HDTMA) to 22.0% (2.0 CEC-HDTMA), and to 100% for the pure surfactant. The fourth mass loss over the temperature from 600 to 720 °C is ascribed to the loss of structural hydroxyl groups from within the clay. The intensity of this peak decreases as the increase of the surfactant concentration and at 2.0 CEC-HDTMA, the fourth mass loss step in the DTG curve is almost disappeared.
From the results, it is noted that there are four steps attributed from DTG figures and Table 1. The dehydration and/or dehydroxylation of adsorbed water from room temperature involves in the first step, and/or the second step under 135 °C includes the dehydration or dehydroxylation of water adsorbed by metal cations. The decomposition of surfactant at the temperature between 263 and 338 °C is observed in the third step. In the last step, the dehydroxylation of structural OH units in the clay is observed.
Conclusions
The organoclays were prepared using MMT and HDTMA as cationic surfactant with long alkyl chain. A combination of XRD and TG is applied in this study to investigate the surfactant loading. Modification of MMT using cationic surfactant into clay interlayer space results in an increase of the total amount of the loaded organic carbon and basal spacings of the organoclays. Based on the basal spacing from the XRD pattern, information of molecular arrangement within the clay interlayer space has been provided as a function of surfactant concentration. The configurations of surfactant within the organoclays take a lateral monolayer arrangement at a lower surfactant concentration (0.25 and 0.50 CEC-HDTMA). The configuration has been changed to pseudotrimolecular layer structure with the increase of surfactant loading at 2.0 CEC-HDTMA. The loading of surfactants into MMT also causes a transformation of clay surface property from hydrophilic to hydrophobic. The loaded surfactant in the interlayer space was further investigated by TG. There are four steps attributed in the DTG. The dehydration and/or dehydroxylation of physically adsorbed water and water molecules around metal cations such as Na+ on exchangeable sites in MMT involves in the first two steps. In the third step, the decomposition of surfactant at the temperature is observed and the intercalated surfactants show higher decomposition temperature when compared to the pure surfactant. The dehydroxylation of structural OH units in the clay is in the last step.
References
Betega De Paiva L, Morales AR, Valenzuela Diaz FR, Organoclays. Properties, preparation and applications. Appl Clay Sci. 2008;42:8–24.
Alther G. Cleaning wastewater: removing oil from water with organoclays. Filtr Sep. 2008;45:22–4.
Alther GR. Removing oils from water with organoclays. J Am Water Works Assoc. 2002;94:115–21.
Carmody O, Frost R, Xi Y, Kokot S. Adsorption of hydrocarbons on organo-clays—implications for oil spill remediation. J Colloid Interface Sci. 2007;305:17–24.
Carrizosa MJ, Calderón MJ, Hermosín MC, Cornejo J. Organosmectites as sorbent and carrier of the herbicide bentazone. Sci Total Environ. 2000;247:285–93.
Carrizosa MJ, Koskinen WC, Hermosin MC, Cornejo J. Dicamba adsorption–desorption on organoclays. Appl Clay Sci. 2001;18:223–31.
Cruz-Guzman M, Celis R, Hermosin MC, Cornejo J. Adsorption of the herbicide simazine by montmorillonite modified with natural organic cations. Environ Sci Technol. 2004;38:180–6.
Park Y, Ayoko GA, Frost RL. Application of organoclays for the adsorption of recalcitrant organic molecules from aqueous media. J Colloid Interface Sci. 2011;354:292–305.
Xi YF, Frost RL, He HP, Kloprogge T, Bostrom T. Modification of Wyoming montmorillonite surfaces using a cationic surfactant. Langmuir. 2005;21:8675–80.
Xi Y, Zhou Q, Frost RL, He H. Thermal stability of octadecyltrimethylammonium bromide modified montmorillonite organoclay. J Colloid Interface Sci. 2007;311:347–53.
Zhou Q, Frost RL, He H, Xi Y, Zbik M. TEM, XRD, and thermal stability of adsorbed paranitrophenol on DDOAB organoclay. J Colloid Interface Sci. 2007;311:24–37.
Zhu L, Chen B, Shen X. Sorption of phenol, p-nitrophenol, and aniline to dual-cation organobentonites from water. Environ Sci Technol. 1999;34:468–75.
He H, Ding Z, Zhu J, Yuan P, Xi Y, Yang D, Frost RL. Thermal characterization of surfactant-modified montmorillonites. Clays Clay Miner. 2005;53:287–93.
Cheng H, Yang J, Frost RL, Liu Q, Zhang Z. Thermal analysis and infrared emission spectroscopic study of kaolinite–potassium acetate intercalate complex. J Therm Anal Calorim. 2011;103:507–13.
Palmer SJ, Grand LM, Frost RL. Thermal analysis of hydrotalcite synthesised from aluminate solutions. J Therm Anal Calorim. 2011;103:473–8.
Bakon KH, Palmer SJ, Frost RL. Thermal analysis of synthetic reevesite and cobalt substituted reevesite (Ni, Co)6Fe2(OH)16(CO3)·4H2O. J Therm Anal Calorim. 2010;100:125–31.
Frost RL, Palmer SJ, Grand L-M. Synthesis and thermal analysis of indium-based hydrotalcites of formula Mg6In2(CO3)(OH)16·4H2O. J Therm Anal Calorim. 2010;101:859–63.
Frost RL, Palmer SJ, Kristof J, Horvath E. Dynamic and controlled rate thermal analysis of halotrichite. J Therm Anal Calorim. 2010;99:501–7.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites containing manganese. J Therm Anal Calorim. 2010;100:981–5.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites based upon gallium. J Therm Anal Calorim. 2010;101:195–8.
Palmer SJ, Frost RL. Thermal decomposition of Bayer precipitates formed at varying temperatures. J Therm Anal Calorim. 2010;100:27–32.
Tao Q, He H, Frost RL, Yuan P, Zhu J. Thermal decomposition of silylated layered double hydroxides. J Therm Anal Calorim. 2010;101:153–9.
Yang J, Frost RL, Martens WN. Thermogravimetric analysis and hot-stage Raman spectroscopy of cubic indium hydroxide. J Therm Anal Calorim. 2010;100:109–16.
Frost RL, Kristof J, Horvath E. Controlled rate thermal analysis of sepiolite. J Therm Anal Calorim. 2009;98:423–8.
Xi Y, Ding Z, He H, Frost Ray L. Structure of organoclays—an X-ray diffraction and thermogravimetric analysis study. J Colloid Interface Sci. 2004;277:116–20.
He HP, Guo JG, Zhu JX, Hu C. 29Si and 27Al MAS NMR study of the thermal transformations of kaolinite from North China. Clay Miner. 2003;38:551–9.
Acknowledgements
This study was supported by the Hungarian Ministry of Culture and Education under grant no. TÁMOP-4.2.2-08/1/2008-0018. The financial and infrastructural support of the State of Hungary and the European Union in the frame of the TÁMOP-4.2.1/B-09/1/KONV-2010-0003 project is gratefully acknowledged. The financial and infra-structure of the Queensland University of Technology, chemistry discipline is gratefully acknowledged. The Australian Research Council (ARC) is thanked for funding some of the instrumentation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, Y., Ayoko, G.A., Kristof, J. et al. A thermoanalytical assessment of an organoclay. J Therm Anal Calorim 107, 1137–1142 (2012). https://doi.org/10.1007/s10973-011-1568-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1568-8