Abstract
The root of Wikstroemia indica has been widely used in China as folk medicine for the treatment for arthritis, whooping cough, cancer, and bacillosis. However, the constituents which have antibacterial activity were not clarified yet. In this study, the antibacterial effect of five extracts from W. indica on Escherichia coli was evaluated by microcalorimetry coupled with agar dilution method. The ethanol extract of W. indica was isolated with organic solvents of different polarities including petroleum (P.E.) extract, chloroform (CHCl3) extract, ethyl acetate (EtOAc) extract, n-butylalcohol (nBuOH) extract, and residue extract. The metabolic profiles of E. coli growth at 37 °C were measured by microcalorimetry. According to the principal component analysis, k 1, k 2, and P 1 were obtained from heat flow power–time (HFP–time) curve. The agar dilution method was performed to verify the results of thermodynamics. The results of microcalorimetric experiment indicated that EtOAc fraction demonstrated the strongest antibacterial activity with half-inhibitory concentration of 92.4 μg mL−1. Meanwhile, similar results were gained from the common method of agar diffusion, which suggested that EtOAc extract could be further developed as antibacterial bioactive fraction of W. indica. Altogether, microcalorimetry is a useful technique to provide sufficient quantitative information and evaluate the antimicrobial effect with its sensitive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wikstroemia indica (L) C.A.Mey. (W. indica) has been applied as a folk medicine for many years in China. According to the recent studies, the effects of W. indica mainly focused on anticancer [1], anti-inflammatory [2], antiviral [3], and antimalarial [4]. Several studies also showed that ethanol extraction and active components of W. indica had an inhibitory effect on bacteria [5]. So the evaluation of pharmacodynamics action including antimicrobial effects of this folk medicine should be studied deeply and widely by useful and sensitive methods.
As a nondestructive and noninvasive tool, microcalorimetry evaluates aerobic and anoxic microbial growth process in a real-time manner, with reproducibility and long-term baseline stability [6, 7]. To increase sensitivity and accuracy, most microcalorimetries generally use “twin instrument” [8]. Among the wide range of microcalorimetry applications, it has been used to study the antimicrobial activities of many drugs and heavy metal on microorganism (including bacteria, cellular organelles, and animal cells) [9–12], and can supply an automatic and continuous metabolic curve to indicate the growth process with ideal living environment of the biosystem. According to the power–time curves, the effects of drugs on microbe growth can provide abundant quantitative and qualitative information to evaluate the antimicrobial activities of drug and screen for novel antibacterial agents. Therefore, microcalorimetry may be regarded as one of the most sensitive tools in the study of bacterial growth.
Due to the bacteria-producing heat, monitoring power changes plays an important role in evaluating antimicrobial activity and has been attracting increasing attention [13, 14]. Monitoring the metabolic activity of microbe to evaluate the antibacterial mode of W. indica had never been explored. Therefore, in this study, the term isothermal microcalorimetry has been performed to evaluate the antibacterial activities of five extract portions of W. indica on E. coli. The heat flow power–time (HFP–time) curves reflect the dynamic changes in the growth process of E. coli quantitatively under the action of agents. Then, principal component analysis (PCA) is carried out on the quantitative parameters obtained from the metabolic profile of E. coli to distinguish five extracts according to their antimicrobial effects. Half-inhibitory concentration of E. coli metabolism of each sample is calculated. Simultaneously, the common method of agar dilution is used to verify the results of thermodynamics. Figure 1 is the experimental flowchart of this study. Above all, this study aims to explore the different antimicrobial effects among five fraction extracts from W. indica.
Materials and methods
Samples and reagents
W. indica was taken from Hunan Province, and the crude materials were authenticated by Prof. Xiao-he Xiao (China Military Institute of Chinese Materia Medica, 302 Military Hospital of China). The air-dried root of W. indica (400 g) was powdered and extracted with 75 % EtOH two times under heat reflux. The filtrates were mixed together and concentrated as extractum which was further extracted by different organic solvents, and five kinds of extraction liquids are low-temperature-dried, grind to powder, and stored at 4 °C for microcalorimetric measurement and agar doubling dilution. The quantity of each extraction is, respectively, P.E. (1.02 g), EtOAC (10.11 g), CHCl3 (1.13 g), nBuOH (6.68 g), and water layer residue (10.85 g).
Strain E. coli [CMCC (B) 44102] was offered by the National Institutes for Food and Drug Control, Beijing 100051, China. Luria–Bertani (LB) culture medium was prepared by 5 g NaCl and 5 g yeast extract, and 10 g peptone was dissolved in 1000 mL distilled water. And medium pH was adjusted to 7.0–7.2, and the culture medium was sterilized in high-pressure steam at 121 °C for 30 min and stored in a refrigerator at 4 °C. Mueller-Hinton (MH) culture medium contained 17.5 g acid-hydrolyzed casein, 1.5 g soluble starch, 6 g beef extract, 13 g agar in 1000 mL deionized water. The culture medium was sterilized in high-pressure steam at 121 °C for 30 min before it was used.
The extraction solvents such as petroleum ether (P.E.; the batch number: 20140402), chloroform (CHCl3; 20140930), ethyl acetate (EtOAc; 20140622), and n-butyl alcohol (n-BuOH; 20140121) were provided by Beijing Chemical Factory (Beijing, China). All chemicals used were of analytical grade. Ultrapure water was obtained from a Milli-Q Plus system (Bedford, MA, USA), fed by pure water from a Millipore Elix system.
Microcalorimetric measurements
Sample solution preparation
About 20 mg powder of water layer residue of W. indica was dissolved with 20 mL LB culture medium and then lautered through 0.22-µm Millipore film to remove the bacteria in the liquid. And the sample solution was yielded at the concentration of 1 mg mL−1 for microcalorimetric measurements. All the operations had been done in aseptic conditions. P.E. extract, EtOAC extract, CHCl3 extract, and nBuOH extract were prepared with the same procedure.
Microcalorimetric measurement
The microcalorimetric experiments were performed on the thermal activity monitor (TAM) air isothermal calorimeter (Thermometric AB, Sweden),which was equipped with eight twin calorimetric channels, of which one side was used for the sample and the other for a static reference. The generated signal was recorded in situ by a computer. The microcalorimetric measurement was taken using the ampoule method and brought to equilibrium temperature overnight in advance. All 20-mL glass ampoules were cleaned and sterilized in high-pressure steam (0.1 MPa) at 121 °C prior to use. Table 1 shows that 3 mL LB culture medium containing E. coli at the cell density of 1 × 106 colony-forming units (CFU)/mL was inoculated in a sterilized 20-mL glass ampoule, and then different volumes (0, 500, 1000, 1500, 2000, 2500, 3000, 3500 µL) of sample solution were put into each ampoule at a final volume of 10 mL [15]. Ampoules were sealed with wax, shaken-up gently, and placed in measuring channels. All the operations had been done in aseptic conditions.
Principal component analysis
Principal component analysis (PCA) is a multivariate and unsupervised pattern recognition method used for analyzing, classifying, and reducing the dimensionality of numerical datasets in a multivariate problem, and allows the representation of the original dataset with a set of new orthogonal variables called principal components (PCs) generated as linear combinations of the original variables [16]. In this paper, PCA was performed to reduce the computation burden and further carried out to search for the main parameters which were obtained by analyzing the HFP–time curves of E. coli growth affected by roots of W. indica extracts using SPSS statistics software (SPSS for Windows 18.0, SPSS).
Antibacterial effects of different extracts from W. indica were evaluated by agar doubling dilution method.
With sterile technique, extracts from W. indica at different concentrations were poured into aseptic plate. The MH agar medium was taken to the plate containing different kinds of extracts with various concentrations and mixed together for solidification; 18 h later, solvent with bacteria was adjusted to the concentration of 105 CFU/mL; same volume of bacteria liquid was inoculated on agar plate containing different extracts at various concentrations. The plates were incubated overnight at 35 °C for 18–24 h. The agar plate containing extracts without bacterial growth was considered as minimum inhibitory concentration (MIC). Each experiment was tested in triplicate.
Results
Metabolic HFP–time curve of E. coli without drugs
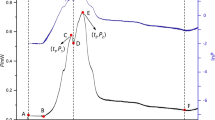
Figure 2 shows the metabolic HFP–time curves of E. coli in medium as control group absence of any substance are measured by microcalorimeter. There are two curves in Fig. 2: the HFP-t curve (the lower one) and the corresponding lnP-t curve (the upper one) of E. coli at 37 °C. The lnP-t curve indicated the changing character of the metabolic heat power and the HFP–time curve indicated the metabolism profile of E. coli which is the typical growth curve for E. coli and is usually divided into six growth phase: the lag phase (I), the first exponential phase (II), the transition phase (III), the second exponential phase (IV), the stationary phase (V), and the decline phase (VI). From the standard curve, P 1, P 2, t 1, t 2, k 1, and k 2, six key thermokinetic parameters were obtained and would be further analyzed. [14, 17].
Since the E. coli was cultured in the ampoule and replication progress was monitored in isothermal and isochoric conditions, the nutrient and oxygen consumed by bacteria were in limited supply. When the bacteria are inoculated into fresh medium, the bacteria exist a lag phase to adapt to new condition (I). Regrettably, it cannot be exactly displayed for the thermal balance in the ampoule method. Because of sufficient nutrient and oxygen, the bacteria enter into the first exponential growth phase (II), which represents the aerobic proliferation metabolism of E. coli [17]. With multiplying rapidly and running out of the oxygen, the bacteria will adjust themselves to adapt to the anaerobic condition and go into a transition phase (II). While plenty of nutrients remain in this growth system, the bacteria will adapt another way to accommodate the growth environment and begin the second exponential growth phase (IV) [18]. In this phase, all the bacteria sufficiently utilize the nutrients to grow actively and multiply swiftly. When the limited nutrients are exhausted, these bacteria will even produce many toxic materials and go into the decline phase (V). When no further bacterial metabolic activity is measurable, power curves get to the baseline and the following dormant phase appears (VI). Therefore, the experiment is finished.
Quantitative thermokinetic parameters for E. coli growth
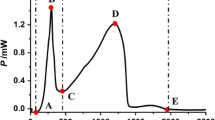
From the metabolic power–time profiles of E. coli growth (Fig. 3), seven quantitative thermokinetic parameters were obtained and are listed in Table 2. And the seven important thermokinetic parameters could reflect the influences of five fractions on the growth of E. coli. P 1 and P 2 (mW) are heat flow powers of first and second highest peaks, and t 1 and t 2 (min) are the corresponding appearance time. And k 1 and k 2 present the metabolism rate constant of the first and the second exponential growth phase for E. coli growth. Then, total heat output (Q) was obtained from the P–t curve of E. coli growth.
During the exponential phase, the P-t curve of E. coli growth could obey the following equation [19]:
where P 0 and P t were the power at initial time and any time, respectively. Thus, using the data lnP t and t taken from the curves to fit a linear equation, k 1 and k 2 represent the metabolism rate constant or growth rate constant of the first and the second exponential phase for E. coli growth under the action of five fractions at different concentrations. We could see that in comparison with control, the growth of E. coli was decreased with the increasing concentration of samples including EtOAc portion, nBuOH portion, and CHCl3 portion, indicating that those portions may have antibacterial effect.
PCA
In order to further show the tendency and internal change rule of different polarities on the effect, correspondence analysis the thermokinetic parameters k 1, k 2, t 1, t 2, P 1, P 2, Q in Table 2 were set as X 1, X 2, X 3, X 4, X 5, X 6, X 7 in correspondence analysis. The result of the PCA showed that the first three principal components (Z 1, Z 2, and Z 3) contained 90.44 % of the information of the original seven indexes. The equation of three principal components showed the distribution of these seven indexes. The equations were:
These relations indicate that parameters k 2, k 1, and P 1 might be the main parameters, which play a more important role in evaluating the antimicrobial effects of W. indica extracts.
Inhibition ratio I and the half-inhibitory concentration IC50 on E. coli
According to the results of PCA, parameters, k 2, k 1, and P 1 might be the focal parameters. By further comparison of k 2, k 1, and P 1 in Table 3, we could see that parameter k 2 contributed more than k 1 and P 1, indicating k 2 played a focal role. Then, based on the main parameter k 2, another important parameter, inhibitory ratio (I), was calculated and could be defined as:
where k 0 is the growth rate constant of the control, k c is the growth rate constant of the second exponential growth phase at inhibitor concentration C. Inhibition ratio I on E. coli at different concentrations of different polarities of W. indica was calculated from Eq. (3). When the inhibitory ratio I is 50 %, the corresponding concentration of inhibitor is expressed as IC50, another necessary parameter to evaluate the activity of antimicrobial agent. Because the inhibitory rate of P.E. fraction did not reach 50 % and residue fraction had no anti-E. coli activity, we only calculated the IC50 of CHCl3, EtOAc, and nBuOH fractions. The sequence of IC50 of the three extracts was EtOAc fraction < CHCl3 fraction < nBuOH fraction. EtOAc fraction had the smallest value of IC50 (92.40 µg mL−1), while nBuOH fraction had the biggest value of IC50 (218.8 µg mL−1) among the three extracts of W. indica (Table 2), which indicated that EtOAc fraction might have the strongest anti-E. coli activity among them.
The result of agar dilution method
The MIC ranges and the 50 % (MIC50s) and 90 % (MIC90 s) MICs for different extracts of W. indica are shown in Table 3. Except for residue part, the sizes of colonies decreased sharply with the concentration of samples on agar plates increasing. And residue extracts had no antibacterial activities. E. coli was susceptible to EtOAc extract (MIC50, 32 mg mL−1; MIC90, 256 mg mL−1), CHCl3 extract (MIC50, 64 mg mL−1), nBuOH extract (MIC50, 64 mg mL−1), and P.E. extract (MIC50, 128 mg mL−1). MIC50 was used for tentatively evaluating the antibacterial activity of the five extracts from W. indica on E. coli. The lower the MIC50, the stronger the inhibitory effect on the E. coli among the five portions in this study. And a potential antimicrobial activity sequence, EtOAc extract > CHCl3 extract/nBuOH extract > P.E. extract was obtained.
Discussion
W. indica is distributed in the southeast of China. It has long been used as a traditional crude drug for the treatment for pneumonia, rheumatism, and antibacterial in China. The antibacterial activity of W. indica has been reported previously, but the constituents that have antibacterial activity have not been clarified yet. [20] In this study, the ethanol extracts of W. indica were fractionated, and various fractions were examined for their activity against E. coli based on microcalorimetry coupled with agar dilution method.
In this study, microcalorimetry was performed due to its high sensitivity and accuracy especially when the concentration of sample was low. From the results of the study, in the power–time curves of E. coli, we could see that in comparison with control, the k 2 of the second exponential phase of E. coli was decreased with the increasing concentration of samples including EtOAc portion, nBuOH portion, and CHCl3 portion, indicating that E. coli decreased the growth speed. Simultaneously, sufficient quantitative parameters were obtained to estimate the antibacterial effects of different extractions of W. indica. Based on PCA, k 1, k 2, P 1 were considered as the main parameters, which played a more important role in evaluating the antibacterial effect of W. indica extraction. Further analyzed, parameters k 2 contributed more than k 2 and P 1, indicating k 2 played a focal role. So, a potential antimicrobial activity sequence, EtOAc extract > CHCl3 extract > nBuOH extract > P.E. extract, of the tested samples was obtained by microcalorimetry. At the same time, in Table 3, similar results were obtained from common pharmacological experiments.
As a useful tool, microcalorimetry is proven to be a higher efficient and valuable tool in several fields of microbiology [8] compared with conventional techniques. IMC microbiology, using the methods that we describe, entails real-time, continuous measurement of microbial metabolic heat produced in sealed ampoules containing growth medium, bacteria, and antibiotic. And it can distinguish bacterial species based on the heat flow power–time curves. One noteworthy environment application is efficient assessment of bacterial activities directly without adding radiolabelled, fluorescent, or chromogenic substrates [6]. For medical application, microcalorimetry is a rapid detection way of bacterial infection or contamination, which is of vital importance in quickly implementing the correct treatment [21].
In summary, our study illustrates that EtOAc extract from W. indica demonstrated stronger antibacterial effects on E. coli growth than each other parts. The antibacterial effects of W. indica were not limited in one active portion, showing that W. indica exerted antibacterial effects by different active components working coordinately in different ways. The W. indica and the actual action mechanism of active compounds on microorganisms need further investigation. This study also illustrated that it was possible for microcalorimetry coupled with agar dilution method to evaluate the antimicrobial effect, which further provided the basis to develop a new method to assess the antibacterial effect of other Chinese material medicas.
References
Kato A, Hashimoto Y, Kidokoro M. (+)-Nortrachelogenin a new pharmacologically active lignan from Wikstroemia indica. J Nat Prod. 1979;42:159–62.
Wang LY, Unehara T, Kitanaka S. Anti-inflammatory activity of new guaiane type sesquiterpene from Wikstroemia indica. Chem Pharm Bull. 2005;53:137–9.
Hu K, Kobayashi H, Dong A, Iwasaki S, Yao X. Antifungal antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica. Planta Med. 2000;66:564–7.
Nunome S, Ishiyama A, Kobayashi M, Otoguro K, Kiyohara H, Yamada H, Omura S. In vitro antimalarial activity of biflavonoids from Wikstroemia indica. Planta Med. 2004;70:76–8.
Yang ZY, Du ZM. Study on antibacterial effect of Wikstroemia indica decoction. Journal of Harbin Pharmaceutical. 2006;40:362–4.
Braissant O, Wirz D, Gopfert B, Daniels AU. Use of isothermal microcalorimetry to monitor mi crobial activities. FEMS Microbiol Lett. 2010;303:1–8.
Wang J, Cheng DH, Zeng N, Xia HL, Fu Y, Yan D, Zhao YL, Xiao XH. Microcalorimetric study of the effect of Benzoinum and Styrax on the growth of Escherichia coli. Nat Prod Res. 2011;25:457–63.
Ding L, Li X, Liu P, Li SQ, Lv JL. Study of the Action of Se and Cu on the Growth Metabolism of Escherichia coli by Microcalorimetry. Biol Trace Elem Res. 2010;137:364–72.
Zheng QF, Zhao YL, Wang JB, Liu TT, Zhang B. Spectrum-effect relationships between UPLC fingerprints and bioactivities of crude secondary roots of Aconitum carmichaelii Debeaux (Fuzi) and its three processed products on mitochondrial growth coupled with canonical correlation analysis. J Ethnopharmacol. 2014;153:615–23.
Zhao YL, Wang JB, Zhang P, Li RS, Xiao XH. Microcalorimetric study of the opposing effects of ginsenosides Rg1 and Rb1 on the growth of mice splenic lymphocytes. J Therm Anal Calorim. 2011;104:357–63.
Dai CM, Wang JB, Peng C, Xiao XH. Investigation of anti-microbial activity of catechin on Escherichia coli growth by microcalorimetry. Environ Toxicol Pharmacol. 2010;30:284–8.
Kong WJ, Jin C, Xiao XH, Zhao YL, Li ZL, Zhang P, Liu W, Li XF. Comparative study of effects of two bile acid derivatives on Staphylococcus aureus by multiple analytical methods. J Hazard Mater. 2010;179:742–7.
Vor T, Dyckmans J, Flessa H, Beese F. Use of microcalorimetry to study microbial activity during the transition from oxic to anoxic conditions. Biol Fert Soils. 2002;36:66–71.
Li X, Liu Y, Wu J, Liang HG, Qu SS. Microcalorimetric study of Staphylococcus aureus growth affected by selenium compounds. Thermochim Acta. 2002;387:57–61.
Liu SX, Zhao YL, Zeng N, Liu TT, Zhang YM, Han B, Li JY, Wang LF, Wang RL, Gong M, Li YG, Xiao XH. Anti-bacterial effect of four extracts from leaves of Dracontomelon dao on Escherichia coli growth using microcalorimetry coupled with principal component analysis. J Therm Anal Calorim. 2014;116:491–7.
Pierce KM, Hope JL, Johnson KJ, Wright BW, Synovec RE. Classification of gasoline data obtained by gas chromatography using a piecewise alignment algorithm combined with feature selection and principal component analysis. J Chromatogr A. 2005;1096:101–10.
Kong WJ, Wang JB, Xing XY, Jin C, Xiao XH, Zhao YL, Zhang P, Zang QC, Li ZL. Screening for novel antibacterial agents based on the activities of compounds on metabolism of Escherichia coli: a microcalorimetric study. J Hazard Mater. 2011;185:346–52.
Li JX, Wang JY, Zhang LL, Yan D, Wang RL, Li BC, Xiao XH. Microcalorimetric investigation on the interaction of six alkaloids from Rhizoma Coptidis. Acta Pharmaceutica Sinica. 2013;48:1807–11.
Xie CL, Tang HK, Song ZH, Qu SS. Microcalorimetric study of bacterial growth. Thermochim Acta. 1988;123:33–41.
Lu CL, Zhu L, Piao JH, Jiang JG. Chemical compositions extracted from Wikstroemia indica and their multiple activities. Pharmaceutical Biol. 2012;50:225–31.
Trampuz A, Salzmann S, Antheaume J, Daniels AU. Microcalorimetry: a novel method for detection of microbial contamination in platelet products. Transfusion. 2007;47:1643–50.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, C., Qu, F., Wang, J. et al. Antibacterial effect of different extracts from Wikstroemia indica on Escherichia coli based on microcalorimetry coupled with agar dilution method. J Therm Anal Calorim 123, 1583–1590 (2016). https://doi.org/10.1007/s10973-015-4999-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4999-9