Abstract
In this study, the combined and simultaneous actions of H2O2 and UV radiation on cassava starch granules were applied and the thermal, rheological, structural and colour properties were investigated. Samples of native cassava starch were oxidised with standard H2O2 solutions (0.1, 0.2 and 0.5 mol L−1) and exposed for 1 h under UV light (UVC radiation with λ = 256 nm), with constant stirring. The solutions were subsequently filtered, washed, dried and analysed. The thermogravimetric curves showed similar behaviour, with three main mass losses and an increase in the thermal stability of each sample. The oxidative modification performed caused a strong decrease in the setback and final viscosity parameters (RVA), a gradual decrease in the gelatinisation enthalpy (DSC) and relative crystallinity (XRD) and significant differences in the average roughness of the granules (NC-AFM). The X-ray diffraction powder patterns displayed the “A” type for all the starch granules. The colour parameters showed a decrease in the −a* value (trend to green) for all the treated samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch is one of the most abundant carbohydrate reserves in plants, and it is the main source of carbohydrates in the human diet [1]. Because it is sourced from renewable resources and is non-toxic, its derivative products have established applications in many fields [2]. In addition to being a major food item, it is used industrially for coatings and sizing in paper, textiles and carpets, and cement and drug delivery systems, as binders and adhesives, absorbents, bone replacement implants, etc. [3].
Cassava root (Manihot esculenta C) is one of the most important starchy tubers in tropical regions. Around the world, 167 million tonnes of fresh tubers and roots are produced annually. Cassava roots are rich in starch: around 90 % of the dry matter. The main composition of the roots is: moisture 70–80 %, starch 16–24 % and <4 % of lipids, proteins, and minerals and vitamins. Cassava root is also used industrially for the production of starch and its derivatives, such as glucose and dextrin [4, 5].
The singular technological properties of cassava starch, such as the non-existence of the typical “cereal flavour” as found in corn and other cereal starches, as well as its capability for higher swelling during cooking and lower pasting temperatures, allow cassava starch to be used in several industrial applications [6].
Starch granules, which are a semi-crystalline polymer, contain two different macromolecules: amylose and amylopectin, which represent approximately 98–99 % of the dry mass. The functionality of starch depends on the average molar masses of amylose and amylopectin, as well as on their molecular structure and the granular organisation [4]. Amylose is an essentially linear polymer with α-d-glucopyranose units connected through α-1-4 linkages. Amylopectin is a highly branched polymer; 5 % of its structure consists of α-1-6 linkages at the branch points [7–9].
Native starches cannot have the physical or chemical characteristics necessary for industrial processing. In these cases, the modifications are therefore necessary. The major percentage of oxidised starches is used in paper industry. The lightly oxidised starches (with H2O2) are used in breading batters for deep-fried foods. Starches can be modified by chemical, physical, enzymatic or combined methods. Oxidative modification has been the subject of much research, mainly on how to develop starch pastes with more fluidity, cohesion, transparency and whiter granules [5, 6, 10, 11].
During the oxidation process, the hydroxyl groups of starch molecules are oxidised to carbonyl groups, followed by the carboxyl groups. The main reagents used for starch oxidation are sodium hypochlorite and hydrogen peroxide, but ammonium persulfate, sodium bromate and potassium permanganate are also used [12].
Hydrogen peroxide is one of the reagents employed to produce oxidised starches and the very complex reactions that occur during starch modification [13]. Unlike sodium hypochlorite, which leaves traces of chlorine in its final product, hydrogen peroxide creates no harmful substances due to the decomposition of oxygen in water. For this reason, hydrogen peroxide is considered to be more environmentally friendly and is mainly chosen when a chlorine-free process is required [14].
The wavelength and intensity of ultraviolet irradiation of starch lead to depolymerisation, which may produce dextrins, mono- and oligosaccharides, water and carbon dioxide [15]. The treatment of starch with UV radiation induces changes in its functional properties, such as increases in water binding capacity and solubility, and decreases in hot paste viscosity [16]. The usage of a combined method, like the one realised by this study (UV radiation + H2O2 solutions), could accelerate the modification process that is required to turn the starch more suitable for industries process, meaning economy of power, chemicals and others.
The present study examined the effects of oxidative modification on native cassava starch for 1 h using standard hydrogen peroxide solutions in concentrations of 0.1, 0.2 and 0.5 mol L−1 and with the simultaneous action of UV radiation (λ = 256 nm). The main properties of the modified and native starches were evaluated by thermal analysis, X-ray diffractometry and rheological and microscopic analysis. Throughout these evaluations, it is possible to present the several characteristics that could be suitable for many industries applications, like the incorporation of starch on paper manufacture [3]. The colour parameters of the untreated and modified starches were also evaluated.
Materials and methods
The native, or untreated, cassava starch used in this study was acquired commercially, in Tozetto Supermarket in October 2013, lot 211-A—expiration date April 2014, Ponta Grossa, PR, Brazil. The starch was divided into four parts of 50 g (dry basis). One part was maintained as it was received, and it was referred to as the (N) native or untreated sample. The other three samples were treated using the methodology [17], with the following modifications: to each native sample (1, 2 and 3, respectively), standard solutions were added at 0.1, 0.2 and 0.5 mol L−1 of H2O2 with constant stirring into a recipient with reflective walls where the samples were exposed to a UV radiation (λ = 256 nm). The samples were maintained in this manner for 1 h. The recipient had a germicide lamp of 9 W (Osram Puritec). The modified samples were subsequently filtered and washed until the complete elimination of the reagent. This test consist in add drops of 0.1 mol L−1 of potassium permanganate solution in the washed water. The samples were dried in an oven with forced air circulation for 24 h at 40 °C, until constant mass and stored in a desiccator.
The thermogravimetric/derivative thermogravimetric (TG/DTG) curves were obtained using a TGA-50 (Shimadzu, Japan) thermal analysis system, where the samples were heated from 35 to 650 °C using open alumina crucibles with approximately 8.0 mg of each sample under a synthetic air flow of 150 mL min−1 at a heating rate of 10 °C min−1. The instrument was preliminarily calibrated with standard mass and with standard calcium oxalate monohydrate. All mass loss percentages were determined using TA-60 WS data analysis software. The derivative thermogravimetric (DTG) curves, the first derivative of the TG curves, were calculated [18–21]. The DSC curves were obtained using a DSC-Q200 (TA-Instruments, USA) thermal analysis system model.
The differential scanning calorimetry (DSC) curves were recorded under an air flow of 50 mL min−1, a heating rate of 10 °C min−1 for a temperature range of the room temperature until 100 °C and the samples weighing about 2.5 mg. A 4:1 (water:starch w/w) mixture was prepared, for each sample, previously on test tubes, and maintained for 60 min in order to equilibrate the moisture content. Then, an aliquot (20 µL) of the samples was transferred with a micropipette into aluminium crucibles and was sealed, and then the curves were recorded. The measurements of “onset” initial temperature (T o), peak temperature (T p), “endset” final temperature (T c) and gelatinisation enthalpy (∆H gel) were taken three times for each sample with the data software Origin 6.0. The instrument was previously calibrated with 99.99 % purity indium, with a melting point of T p = 156.6 °C, ∆H = 28.56 J g−1 [18–20, 22].
The pasting properties of the samples were obtained using a rapid viscoamylograph—RVA-4 (Newport Sci., Australia). A suspension of 3.0 g of starch in 25.0 g of distilled water underwent a controlled heating and cooling cycle under constant shear, where it was held at 50 °C for 2 min, heated from 50 to 95 °C at 6 °C min−1, held at 95 °C for 5 min, cooled to 50 °C at 6 °C min−1 and held at 50 °C for 2 min [19, 20]. The micro-images of each sample were observed with high resolution using a SPM-9600 atomic force microscope (Shimadzu, Japan), employing the non-contact method (NC-AFM). All the measurements were taken in triplicate, and this technique allowed us to observe the surface of the studied starches. It was possible to calculate the average diameter and the average roughness of the samples [18–20, 22, 23].
X-ray diffraction powder patterns (XRD) were obtained by using an Ultima 4 (Rigaku, Japan) X-ray diffractometer, employing Cu Kα radiation (λ = 1.541 Å) and settings of 40 kV and 20 mA. The scattered radiation was detected in the angular range of 5–50° (2θ), with a scanning speed of 8° min−1 and a step of 0.06°. The degree of relative crystallinity was calculated using Eq. 1 and following the method described in the literature [18, 20, 23].
where X c, relative crystallinity, A p, peak area, A b, base area.
To determine the colour parameters of the starch, before and after treatment with the H2O2 solutions and UV radiation, a Mini Scan XE 45/0-L Plus (Hunter Inc., USA) reflectance spectrophotometer was used, which consists of three colour components: L*, a* and b*. The colour of the untreated and modified cassava starch samples was evaluated by the following parameters: L*, brightness ranging from 0 (black) to 100 (white); a* ranging from positive (red) to negative (green); and b*, which varies from positive (yellow) to negative (blue) [19, 20].
The normality of the experimental data was evaluated by the Shapiro–Wilk test (p > 0.05 was considered normal), and homogeneity of variance was evaluated by the Brown–Forsythe test (p > 0.05 was considered parametric). The parametric data were evaluated by analysis of variance (ANOVA) complemented with Fisher’s LSD post hoc test. A value of p < 0.05 was considered significant [24]. Statistical analyses were performed using Statistica software, version 8.0 (Statsoft, Tulsa OK, USA).
Results and discussion
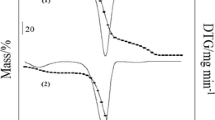
The TG/DTG curves were recorded to study the untreated sample (N) and the modified samples (1, 2 and 3) of cassava starch. TG curves can be helpful to show the behaviour of starch granules [18–22, 25]. The profile of each curve showed three main mass losses, and three distinct regions were observed on the TG/DTG curves. The first region began at room temperature up to around 160 °C, which represented the evaporation of the water and volatile compounds, followed by stability.
Once dehydrated, the second and third main regions in the TG curves were due to the degradation of the organic matter, with the formation of final residue (ash), which was around 1 % of the initial mass of each sample, respectively. All the TG/DTG curves are shown in Fig. 1, and the results are given in Table 1.
In Table 1, the peak temperature (T p / °C) values for the second events presented for sample (N) was 362.61, sample (1) 361.49, sample (2) 359.09, sample (3) 358.36. By these results, it can be said, according to the literature [26, 27], that the decrease in the results for the modified samples comparing with the native one is due to the decrement of molecular mass of the starch during the thermal degradation.
A similar profile of thermogravimetric curves was reported in an earlier study of cassava starch granules treated with standard hydrogen peroxide at 1, 2 and 3 % in the presence of a standard solution of FeSO4 at 0.01 % [8]; differences between the decomposition temperatures were attributed to the different treatments of the cassava starches.
The results showed that the modified starches had their stability step values shifted to higher temperatures, in comparison with the untreated sample. The DSC curves were recorded in order to study the gelatinisation process. Figure 2 shows the profile of the DSC curves.
Table 2 gives the DSC results, and it can be seen that, compared with the (N) native cassava starch sample, the peak temperatures (T p) of the modified samples did not show significant differences. The reduction in peak temperatures (T p) depends on the moisture level of the treatment, the starch source and the amylose content [28–30]. The calculated gelatinisation enthalpy (∆H gel) presented an increase, contrasting with the result observed for sample (3) and characterising that the modification occurred in this sample. Similar values for enthalpy were found in the literature [18–20]: 10.98, 13.44, 11.45 and 13.45 J g−1, respectively, for cassava starch treated with hydrochloric acid at different temperatures; for cassava starch treated in a dual heat–moisture treatment; for cassava starch treated with heat–moisture; and for cassava starch exposed to microwave radiation. The differences between the results of ∆H gel were due to the different treatments that were performed (reagent concentrations, time of treatment, genetic varieties and physical methods of treatments).
The profile of the RVA curves and the pasting properties of the studied starches are shown in Fig. 3, and the results are given in Table 3. During oxidation processes, the hydroxyl groups in starch molecules are oxidised to carbonyl and carboxyl groups, which contributes to improved stability in starch paste [14]. This can be observed in the pasting temperature results for the samples (shown in Table 3) which demonstrate significant difference for the treatment of sample (3). Regarding the viscosity peaks, it was observed that there was a strong decrease for the oxidised samples compared with the untreated samples: sample (N) 3870 cP; sample (1) 2016 cP; sample (2) 1886 cP (the lowest value); and sample (3) 1960 cP. This phenomenon occurs because during the oxidation process, the hydroxyl groups of the starch molecules are oxidised to carbonyl groups, followed by the carboxyl groups altering their molecular structure and properties [12]. The modification of starches by UV radiation could induce the alteration in their main properties [31]. It could promote the faster bound of the molecules of starch with the water, increasing the starch solubility [32] and also decreasing the starch paste viscosity [33], as observed in this study.
RVA curves of: (N) native cassava starch; (1) native cassava starch treated by 60 min with UV-radiation (λ = 256 nm) and H2O2 standard solution 0.1 mol L−1; (2) native cassava starch treated by 60 min with UV-radiation (λ = 256 nm) and H2O2 standard solution 0.2 mol L−1; (3) native cassava starch treated by 60 min with UV-radiation (λ = 256 nm) and H2O2 standard solution 0.5 mol L−1, respectively
Similar behaviour regarding RVA profiles was reported in previous studies of treated cassava starches for the pasting temperatures and viscosity peaks [12, 20]. Nevertheless, lower values were observed for setback and final viscosity for each sample in the present study. The food industry requires starches with low setback values, which are linked with a lower tendency for retrogradation. The main influence of the retrogradation process is on the texture, acceptability and digestibility of starchy processed food; it affects the deterioration of some products, such as bread and other bakery products, and water loss (syneresis) in starchy deserts [34]. Similar behaviour was found in studies of the thermal, rheological and structural properties of natural cassava starch, and cassava starch modified with hydrochloric acid (HCl) and with sodium hypochlorite NaClO [5, 18], respectively.
The micro-images of the treated and untreated cassava starch granules shown in Fig. 4 were obtained by the non-contact method of atomic force microscopy (NC-AFM). As can be seen, the micro-images of the starch granules of the samples presented slight differences between the (N) samples and the treated ones, especially in terms of depressions and protrusions. The particle dimension distribution, granule morphology and the surface uniqueness of starch granules play an important function in many of the food and non-food applications of starch [20].
The average diameter (d a) and average roughness (r a) were calculated, and the results are given in Table 2. The results presented significant differences between the samples in terms of roughness and no significant differences regarding the average diameters between the samples (N), (1) and (3). These results are in agreement with the literature [1], which reports that the treatment of cassava starch with hydrogen peroxide does not considerably alter the shape and size of starch granules.
The X-ray diffractograms demonstrated that the modified starches showed changes in crystallinity, as can be seen in Fig. 5. Previous research [35, 36] has suggested that starch granules present the following categorisation, depending on their main peak diffractogram patterns: type “A” when the main peaks are shown in 15°, 17°, 18° and 23° into 2θ; type “B” in 5°, 6°, 15°, 17°, 18° and 23° into 2θ; and type “C” is considered to be a mix between the values 5.5°, 15°, 17°, 22° and 23° into 2θ. An earlier study proposed that starches tend to present pertinent crystalline arrangements depending on their botanical origin [1]. Many of the tuberous starches, such as cassava starch, are classified as “A” or “C” or even “AC” [30].
XRD diffractogram of: (N) native cassava starch; (1) native cassava starch treated by 60 min with UV-radiation (λ = 256 nm) and H2O2 standard solution 0.1 mol L−1; (2) native cassava starch treated by 60 min with UV-radiation (λ = 256 nm) and H2O2 standard solution 0.2 mol L−1; (3) native cassava starch treated by 60 min with UV-radiation (λ = 256 nm) and H2O2 standard solution 0.5 mol L−1, respectively
In the present study, the main diffraction peaks occurred at approximately 2θ 15°, 17°, 18° and 23° and had no displacements, which represents the characteristic “A” pattern of cereal starches [30, 35, 36]. The calculated degrees of relative crystallinity (shown in Table 2) using Eq. 1 [19, 20] were 24.53, 20.42, 20.92 and 22.17 % for the samples (N), (1), (2) and (3), respectively. A decrease in crystallinity for the treated samples was observed compared to the (N) sample. Starch is composed of amylose and amylopectin being this last region more susceptible to oxidation process. Similarly behaviour occurs with enzymatic hydrolysis [37].
Table 4 shows the colour parameters for the native and treated samples. Colour is an aspect that has a large effect on consumer perceptions regarding the quality of food products, and it has a major bearing on the acceptance or rejection of such products [38].
In this study, three colour parameters related to the samples were evaluated: L*, a* and b*. The L* value relates to brightness, ranging from white (L* = 100) to black (L* = 0). The a* value represents the colour in the region of red (+a*) to green (−a*). The b* value indicates the colour range from yellow (+b*) to blue (−b*) [5]. Table 4 shows that a difference in the L* value (trend to white) occurred between the untreated sample and the treated samples for the b* value (trend to yellow). There were significant differences between the treated samples and the (N) sample but not between them. However, the −a* (trend to green) value decreased from the untreated sample (N) to the treated sample (3), presenting no significant differences when compared to samples (2) and (1).
Conclusions
The combined and simultaneous actions of H2O2 oxidation and UV light on cassava starch granules had effects on its main properties. The TG/DTG curves for the treated samples showed an increase in the thermal stability for the treated samples, except for sample (3), which showed a slight shift in decomposition temperatures. The DSC profiles presented a gradual decrease in the gelatinisation enthalpy, except for sample (3), which presented a slight increase. The RVA analysis showed strong decreases in the setback and final viscosity results. No significant change in the average diameter of the granules was obtained by using the NC-AFM technique, except for sample (1), and XRD showed that the degree of relative crystallinity decreased for the treated samples. Reflectance spectroscopy showed a considerable decrease in the tendency to green for sample (3).
References
Costa FJOG, Leivas CL, Waszczynsky N, Godoi RCB, Helm CV, Colman TAD, Schnitzler E. Characterisation of native starches of seeds of Araucaria angustifolia from four germplasm collections. Thermochim Acta. 2013;565:172–7.
Hou C, Chen Y, Chen W, Li W. Microwave-assisted methylation of cassava starch with dimethyl carbonate. Carbohydr Res. 2011;346:1178–81.
Neelam K, Vijay S, Lalit S. Various techniques for the modification of starch and the application of its derivatives. Int Res J Pharm. 2012;3:25–31.
Jankovíc B. Thermal characterization and detailed kinetic analysis of Cassava starch thermo-oxidative degradation. Carbohydr Polym. 2013;95:621–9.
Beninca C, Colman TAD, Lacerda LG, Carvalho-Filho MAS, Bannach G, Schnitzler E. The thermal, rheological and structural properties of cassava starch granules modified with hydrochloric acid at different temperatures. Thermochim Acta. 2013;552:65–9.
Garrido LH, Schnitzler E, Zortéa MEB, Rocha TS, Demiate IM. Physicochemical properties of cassava starch oxidized by sodium hypochlorite. J Food Sci Technol. 2012;. doi:10.1007/s13197-012-0794-9.
Beninca C, Demiate IM, Lacerda LG, Carvalho-Filho MAS, Ionashiro M, Schnitzler E. Thermal behaviour of corn starch granules modified by acid treatment at 30 and 50 °C. Ecl Quim. 2008;3:8–13.
Costa FJOG, Almeida RR, Lacerda LG, Carvalho-Filho MAS, Bannach G, Schnitzler E. Thermoanalytical study of native cassava starch and treated with hydrogen peroxyde. Alim Nutr. 2011;22:7–15.
Oliveira CS, Andrade MMP, Colman TAD, Costa FJOG, Schnitzler E. Thermal, structural and rheological behaviour of native and modified waxy corn starch with hydrochloric acid at different temperatures. J Therm Anal Calorim. 2014;115:13–8.
Wani IA, Jabeen M, Geelani H, Masoodi FA, Saba I, Muzaffar S. Effect of gamma irradiation on physicochemical properties of Indian horse chestnut (Aesculus indica Colebr.) starch. Food Hydrocoll. 2014;35:253–63.
El-Sheikh MA, Ramadan MA, El-Shafie A. Photo-oxidation of rice starch II. Using a water soluble photo initiator. Carbohydr Polym. 2009;78:235–9.
Klein B, Vanier NL, Moomand K, Pinto VZ, Colussi R, Zavareze ER, Dias ARG. Ozone oxidation of cassava starch in aqueous solution at different pH. Food Chem. 2014;155:167–73.
Matsuguma LS, Lacerda LG, Schnitzler E, Carvalho-Filho MAS, Franco CML, Demiate IM. Characterization of native and oxidized starches of two varieties of peruvian carrot (Arracacia xanthorriza, B.) from two production areas of Paraná state, Brazil. Braz Arch Biol Technol. 2009;3:701–13.
Sangseethong K, Termvejsayanon N, Sriroth K. Characterization of physicochemical properties of hypochlorite- and peroxide-oxidized cassava starches. Carbohydr Polym. 2010;82:446–53.
El-Sheikh MA, Ramadan MA, El-Shafie A. Photo-oxidation of rice starch. Part I: using hydrogen peroxide. Carbohydr Polym. 2010;80:266–9.
Fiedorowicz M, Tomasik P, Sangguan Y, Seung-Taik L. Molecular distribution and pasting properties of UV-irradiated corn starches. Starch/Stärke. 1999;51:126–31.
Dias ARG, Zavareze R, Helbig E, Moura FA, Vargas CG, Ciacco CF. Oxidation of fermented cassava starch using hydrogen peroxide. Carbohydr Polym. 2011;86:185–91.
Beninca C, Colman TAD, Lacerda LG, Carvalho-Filho MAS, Demiate IM, Schnitzler E. Thermal, rheological and structural behaviours of natural and modified starch granules, with sodium hypochlorite solutions. J Therm Anal Calorim. 2013;111:2217–22.
Colman TAD, Demiate IM, Schnitzler E. The effect of microwave radiation on some thermal, rheological and structural properties of cassava starch. J Therm Anal Calorim. 2014;115:2245–52.
Andrade MMP, Oliveira CS, Colman TAD, Costa FJOG, Schnitzler E. Effects of heat moisture treatment on organic cassava starch. Thermal, rheological and structural study. J Therm Anal Calorim. 2014;115:2115–22.
Lacerda LG, Carvalho-Filho MAS, Demiate IM, Bannach G, Ionashiro M, Schnitzler E. Thermal behaviour of corn starch granules under action of fungal α amylase. J Therm Anal Cal. 2008;93:445–9.
Alberton C, Colman TAD, Souza JA, Oliveira CS, Andrade MMP, Schnitzler E. J Microbiol Biotechnol Food Sci. 2014;3:305–9.
Zhang L, Xie W, Zhao X, Liu Y, Gao W. Study on the morphology, crystalline structure and thermal properties of yellow ginger starch acetates with different degrees of substitution. Thermochim Acta. 2009;495:57–62.
Granato D, Calado VMA, Jarvis B. Observations on the use of statistical methods in Food Science and Technology. Food Res Int. 2014;55:137–49.
Aggarwal P, Dollimore D. A thermal analysis investigation of partially hydrolyzed starch. Thermochim Acta 1998;329:17–25.
Xingxun L, Long Y, Fengwei X, Ming L, Ling C, Xiaoxi L. Kinetics and mechanism of thermal decomposition of cornstarches with different amylose/amylopectin ratios. Starch/Stärke. 2010;62:139–46.
Xingxun L, Yanfei W, Long Y, Zhen T, Ling C, Hongsheng L, Xiaoxi L. Thermal degradation and stability of starch under different processing conditions. Starch/Stärke. 2013;65:48–60.
Zavareze ER, Dias ARG. Impact of heat-moisture treatment and annealing in starches: a review. Carbohydr Polym. 2011;83:317–28.
Gunaratne A, Hoover R. Effect of heat–moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydr Polym. 2002;49:425–37.
Shin SI, Kim HJ, Ha HJ, Lee SH, Moon TW. Effect of hydrothermal treatment on formation and structural characteristics of slowly digestible non-pasted granular sweet potato starch. Starch/Stärke. 2005;57:421–30.
Bertolini AC, Mestres C, Colonna P. Rheological properties of acidified and UV irradiated starches. Starch/Stärke. 2001;52:340–4.
Gholap AV, Marondeze LH, Tomasik P. Dextrinization of starch with nitrogen laser. Starch. 1993;45:430–2.
Fiedorowicz M, Tomasik P, Sangguan Y, Seung-Taik L. Molecular distribution and pasting properties of UV-Irradiated corn starches. Starch/Starke. 1999;51:126–31.
Denardin CC, Silva LP. Estrutura dos grânulos de amido e sua relação com propriedades físico-químicas. Cienc Rural. 2009;39:945–54.
Marcon MJA, Kurtz DJ, Raguzzoni JC, Delgadillo I, Maraschin M, Soldi V, Reginatto V, Amante ER. Expansion properties of sour cassava starch (polvilho azedo): variables related to its practical application in bakery. Starch/Stärke. 2009;61:716–26.
Hoover R. Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr Polym. 2001;45:253–67.
Malucelli LC, Lacerda LG, Carvalho-Filho MAS, Rodríguez-Fernández DE, Demiate IM, Oliveira CS, Schnitzler E. Porous waxy maize starch: thermal, structural and viscographic properties of modified granules obtained by enzyme treatment. J Therm Anal Calorim. 2015;120:525–32.
Tuberoso CIG, Jerkovic I, Sarais G, Congiu F, Marijanovic Z, Kus PM. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE L* C*ab h°ab chromaticity coordinates. Food Chem. 2014;145:284–91.
Acknowledgements
The authors gratefully acknowledge the financial resources for this study provided by CAPES-Brazil and CNPq-Brazil and also C-LABMU—UEPG for performing the instrumental analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hornung, P.S., de Oliveira, C.S., Lazzarotto, M. et al. Investigation of the photo-oxidation of cassava starch granules. J Therm Anal Calorim 123, 2129–2137 (2016). https://doi.org/10.1007/s10973-015-4706-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4706-x