Abstract
Organic foods and crops are produced throughout the world under strict controls on growing conditions, so that synthetic chemicals, irradiation or genetic modifications are avoided. Organic starch is extracted following the same rules. Heat–moisture treatment (HMT) on starch is a physical method considered to be natural: it consists of heating starch at a temperature above its gelatinisation point with insufficient moisture (<35 %) to cause gelatinisation. Samples of organic cassava starch (with 12.8 % moisture) were dried in an oven with forced air circulation at 50 °C for 48 h and, immediately, distilled water was added to each sample until it reached the ratios of 10, 20, and 30 %, respectively. The samples were transferred into 100 mL pressure flasks, sealed tightly with a cap, and maintained in an autoclave for 60 min at 120 °C. The flasks were opened and the samples were kept in a desiccator containing anhydrous calcium chloride up to constant mass. The effects of HMT were studied using the following techniques: thermogravimetry and derivative thermogravimetry (TG/DTG), differential scanning calorimetry (DSC), rapid viscoamylographic analysis (RVA), reflectance photocolorimetry, atomic force microscopy (NC-AFM) and X-ray diffractometry (XRD). Rheological properties such as the pasting temperature (RVA) and the peak temperature (DSC) increased, while gelatinisation enthalpy ∆H (DSC) decreased. The average diameters of the granules showed no significant changes, while the degree of relative crystallinity decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cassava (Manihoc esculenta, Crantz) is a species of the Euphorbiaceae family and one of the most important root crops in tropical regions in terms of food energy production [1].
Starch is arguably one of the most actively investigated biopolymers in the world. It is a raw material of various botanical origins and it is the most important carbohydrate reserve in plants: it is used by the food, paper, chemical, pharmaceutical and textile industries, among many others. This biopolymer is the main source of carbohydrates in the human diet due to its abundance in nature, where it is present in the seeds, roots and stems of different plants. Starch granules are made up of glucose polymers, named amylose and amylopectin, which are found inside vegetable cells, and are extracted for industrial applications in the food, textile and paper industries, etc. Amylose is predominantly a linear polymer of α-1,4 linked glucose, whereas amylopectin is a highly branched polysaccharide consisting of α-1,4 linked glucose with α-1,6 linkages at the branch points [2–4].
Organic foods are produced using methods which do not involve modern synthetic inputs such as synthetic pesticides and chemical fertilisers: they do not contain genetic modifications and are not processed using irradiation, industrial solvents or chemical food additives. Several countries require producers to obtain a special certification to market food as ‘organic’ [5, 6].
Organic starch is extracted following the same rules, and for this study, it was kindly supplied by Tozan Co., Ltd., Ponta Grossa, PR, Brazil.
Starches are of great value for the food industry because they contribute greatly to the textural properties of many foods and are used in food and industrial applications as a thickener, colloidal stabiliser, gelling agent, bulking agent and water retention agent [7].
The selection of starches for industrial uses is made considering its availability and also its physicochemical characteristics which can vary depending on the source. Due to this, each natural or native starch property is considered exclusively [8].
Starch, in its native form, does not always have the physical or chemical properties appropriate for certain types of processing. Some of these constraints include insolubility in cold water, low stability to freeze–thawing and synaeresis, which in some cases makes them difficult to use [9, 10].
Starch modifications can be made by chemical, enzymatic and physical methods which promote specific functional properties. The heat–moisture treatment (HMT) of starch is a physical modification that changes the physicochemical properties of starches without destroying its granular structure. This technique is considered to be natural and safe when compared to other modifications and consists on heating starch at a temperature above its gelatinisation point (90–120 °C) with insufficient moisture content (<35 %) to cause the gelatinisation process [11–18].
In this research, the main objective was to study untreated organic cassava starch, as well as organic cassava starch treated with controlled moisture content (10, 20 and 30 %) at 120 °C for 1 h, using the following techniques: thermogravimetry and derivative thermogravimetry (TG/DTG), differential scanning calorimetry (DSC), rapid viscoamylographic analysis (RVA), reflectance photocolorimetry, atomic force microscopy (NC-AFM) and X-ray diffractometry (XRD).
Materials and methods
Organic cassava starch samples were supplied by Tozan Co., Ltd., Ponta Grossa, PR, Brazil.
An aliquot of 40 g of organic cassava starch was divided into four portions of 10 g, which were identified as samples a, b, c and d, and all were kept in a desiccator containing anhydrous calcium chloride up to constant mass. All the samples showed moisture content around 12.8 %.
Sample a was named ‘untreated sample’ and maintained in a desiccator. The other samples were dried in an oven with forced air circulation at 50 °C for 48 h. Immediately after this time, distilled water was added to samples b, c, and d until they reached the ratios of 10, 20, and 30 %, respectively.
After homogenisation, samples b, c, and d were transferred into three 100 mL pressure flasks, sealed tightly with a cap, and maintained in an autoclave for 60 min at 120 °C. After this time, the flasks were opened and the samples were kept in a desiccator containing anhydrous calcium chloride up to constant mass.
The thermogravimetric curves (TG) were obtained using the thermal analysis system TGA-50 (Shimadzu, Japan), where the samples were heated from 25 to 600 °C using open alumina crucibles with approximately 7.0 mg of each sample under a synthetic air flow of 150 mL min−1 at a heating rate of 10 °C min−1. The instrument was preliminarily calibrated with mass standard and with standard calcium oxalate monohydrate. All percentages of mass loss were determined using TA-60 WS data analysis software. The derivative thermogravimetric curves (DTG) were the first derivative of TG curve that were calculated and used in the determination of the mass loss points.
The DSC curves were obtained using a thermal analysis system model DSC-Q200 (TA-Instruments, USA) in two modalities.
The first modality was carried out to follow the thermogravimetric process, according the instrumental conditions: the samples were heated from 25 to 600 °C using an aluminium crucible with perforated cover and approximately 5.0 mg of each sample, under a synthetic air flow of 150 mL min−1 at a heating rate of 10 °C min−1.
In the second modality, the DSC curves were recorded with the aim of studying the gelatinisation process: the curves were recorded under an air flow of 50 mL min−1, heating rate of 10 °C min−1 and samples weighing about 3.5 mg. A 4:1 (water:starch w/w) mixture was prepared and maintained for 60 min to equilibrate the moisture content. The aluminium crucibles were sealed and carried out to study the gelatinisation process. The instrument was previously calibrated using Indium 99.99 % purity, m.p. = 156.6 °C, ∆H = 28.56 J g−1.
The pasting properties of the samples were determined using rapid viscoamylographic analysis RVA-4 (Newport Sci., Australia). A suspension of 3 g (8 % moisture) of starch in 25 g of accurately distilled water underwent a controlled heating and cooling cycle under constant shear, where it was held at 50 °C for 2 min, heated from 50 to 95 °C at 6 °C min−1, and held at 95 °C for 5 min, cooled to 50 °C at 6 °C min−1 and held at 50 °C for 2 min.
At the beginning of the heating the swelling of the starch granules occurs, which causes an increase in the viscosity slurry (time and temperature pasting) when the polymers with low molecular mass (amylose molecules) begin to be leached from the granules. The viscosity peak was observed when the granules were completely swollen and while maintaining the pasting temperature at 95 °C under constant agitation. The granules began to break down and solubilisation of the polymers provided a reduction of viscosity (break). Analysis followed, with a cooling cycle that caused the reorganisation of some amylose and amylopectin polymers increasing the opacity and viscosity of the paste in a process called setback [4, 10], which occurs due to the strong tendency to form hydrogen bonds between adjacent molecules.
The micro-images of each sample, was observed with high resolution using an Atomic Force Microscope SPM-9600 (Shimadzu, Japan), using the non-contact method (NC-AFM). The technique allowed us to observe the surface of the studied starches and it was possible to calculate the average diameter (d a) and the average roughness (r a) of the samples [2, 4, 19–21].
X-ray diffraction powder patterns (XRD) were obtained using an X-ray diffractometer, model Ultima 4 (Rigaku, Japan), employing Cu Kα radiation (λ = 1.541 Å) and settings of 40 kV and 20 mA. The scattered radiation was detected in the angular range of 5°–50° (2θ), with scanning speed of 8° min−1 and a step of 0.06°.
The degree of relative crystallinity was quantitatively estimated, following the method described in the literature [4, 22]. A smooth curve, with connect peak baselines, was computed and plotted on the diffractograms.
The area above the smooth curve was taken as the crystalline portion, and the lower area between the smooth curve and the linear baseline which covers the 2θ range from 5° to 50° was taken as the amorphous section. The upper diffraction peak area and the total diffraction area over the diffraction angle 5°–50° were integrated. The ratio of upper area to total diffraction was used as the degree of relative crystallinity. The equation for calculating the degree of relative crystallinity is as follows:
where X c refers to the degree of relative crystallinity, A p refers to the crystallised area on the X-ray diffractogram and A b refers to the amorphous area on the X-ray diffractogram [1, 4, 10, 22–24].
For determining the colour parameters of the starch, before and after HMT, the MiniScan XE reflectance spectrophotometer 45/0-L Plus (Hunter Inc., USA) was used, which consists of three colour components: L*, a* and b*. The colour of untreated and treated cassava starch samples was evaluated by the parameters L*, brightness ranging from 0 (black) to 100 (white); a* ranging from positive (red) to negative (green); and b*, which varies from positive (yellow) to negative (blue) [4, 25].
All the analyses were made in triplicate. Analysis of variance (ANOVA) and Tukey’s test were used to compare sample means at 95 % confidence level (p < 0.05) using STATISTICA 7.0 software (StatSoft, Inc., Tulsa, OK, USA).
Results and discussion
The untreated sample a and the treated samples with 10 % moisture b, with 20 % moisture c and with 30 % moisture d were maintained in a desiccator with anhydrous calcium chloride until constant mass. After this procedure, the samples were analysed by the conventional and instrumental techniques. The moisture content was determined by gravimetric method and the results were: 12.80, 8.79, 14.51, and 15.20 %, respectively. The results were compatible with those obtained by TG/DTG and are shown in Table 1.
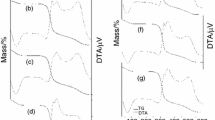
The profile of TG/DTG curves of the studied compounds observed in Fig. 1a–d (left) were similar, with three mass losses. The first mass loss was due to the dehydration process that occured in a single step.
After dehydration, the anhydrous compounds were stable until 152, 161, 166, and 161 °C, respectively, when the second and third mass losses occurred in two consecutive steps that were attributed to the decomposition of organic matter with the formation of ash. The initial mass of each sample was: a 6.52 mg, b 7.92 mg, c 7.83 mg, and d 8.22 mg and the final residue (ash) was 0.19, 0.14, 0.21, and 0.16 % (TG/DTG), of initial mass, respectively. DTG curves were used in the determination of the temperatures and mass losses in TG curves and these results are presented in Table 1.
The DSC curves shown in Fig. 1a–d (right) were carried out to monitor the thermal decomposition process of the samples and to display endothermic (dehydration) or exothermic (decomposition of organic matter) events, results of which are shown in Table 2. The onset temperature (T o), peak temperature (T p), and conclusion temperature (T c) as well as the enthalpy (∆H) of main peaks were calculated and all the experiments were performed in triplicate. After the stability and before the oxidation of organic matter the dextrinisation process occurs, which appears as endothermic peaks near 290 °C. All these results are shown in Table 2.
Figure 2 shows the DSC curves of untreated cassava starch (a) and after treatment for 1 h at 120 °C with 10, 20 and 30 % moisture (b–d), respectively. Each sample was prepared in 4:1 mixture (water:starch, w/w) and maintained for 60 min to equilibrate the moisture content. After this time, the experiment was conducted with the purpose of verifying the gelatinisation parameters.
The onset temperature (T o), peak temperature (T p), conclusion temperature (T c) and gelatinisation enthalpy (∆H gel) were determined and the results are depicted in Table 2. According to the literature [12, 26], these effects are dependent on the moisture level of the treatment, the starch source, and the amylose content. It can be verified that in relation to the untreated starch sample a the peak temperature (T p) initially decreased sample b followed by increases that were directly proportional sample c, d, whereas the gelatinisation enthalpy (∆H gel) decreased. Similar behaviour was observed by [26] who worked with cassava starch heated for 10 h at 100 °C using an oven with forced air circulation.
The profile of RVA curves and pasting properties of the studied starches are shown in Fig. 3 and the obtained results in Table 3.
According to [12–14], HMT promotes changes in starches, significantly altering their pasting profile. The obtained results of the present study are shown in Fig. 3 and Table 3. It can be observed that the pasting temperature increased and the viscosity peak decreased for samples a–c and increased for sample d.
In Fig. 4, with the NC-AFM technique, slight visual differences between the untreated and treated starch granules can be verified, especially when depressions and protrusions were observed on the surface of each sample. It became possible to determine the average particle diameter (d a), which showed no significant difference.
Granule morphology and granule size distribution, and surface characteristics play an important role in many food and non-food applications of starch. The obtained results are in agreement with the literature [12, 26], which reports that HMT does not alter the shape and size of starch granules.
The obtained results regarding average diameter and average roughness are shown in Table 4.
According to the literature [24], crystallinity differences between starches may be attributed to the following factors: the crystal size; the number of crystalline regions that are influenced by amylopectin content and chain length; the orientation of double helices within the crystalline area; and the extent of interactions between the double helices. However, these authors conclude that the effect of HMT on the crystallinity of starches depends on the starch source and treatment conditions.
Figure 5 shows the obtained diffractograms of each sample. No significative displacement occured between the main peaks, and the relative crystallinity was calculated.
As can be observed in Table 4, the degree of relative crystallinity decreased inversely in proportion to moisture content and with HMT.
Visually, there were no observable changes in the colour of the HMT treated samples. However, the colour results obtained by reflectance spectrophotometry indicate that there is a slight trend to yellowing (b*) of the samples, and sample c showed higher intensity.
Conclusions
The moisture content of starches before and after HMT, when maintained in a desiccator in an anhydrous atmosphere trends to present a moisture characteristic according to the treatment provided.
The TG/DTG curves shown similar behaviour characteristic of starches with small changes in ∆H and ∆T. The DSC profile showed a gradual decrease in gelatinisation enthalpy with changes in the onset and peak temperatures of each sample; the RVA results were proportional to DSC results except for sample c.
The NC-AFM technique showed no significant change in the average diameter of the granules, and XRD showed that the degree of relative crystallinity decreased for the samples with higher moisture content and the reflectance spectroscopy shown slight trend to yellowing.
References
Costa FJOG, Almeida RR, Lacerda LG, Carvalho-Filho MAS, Bannach G, Schnitzler E. Thermoanalytical study of native cassava starch and treated with hydrogen peroxide. Aliment e Nutrição. 2011;22:7–15.
Leivas CL, Costa FJOG, Almeida RR, Freitas RJS, Stertz SC, Schnitzler E. Structural characteristics, physico-chemical, thermal and pasting properties of potato (Solanum tuberosum L.) flour: study of different cultivars and granulometries. J Therm Anal Calorim. 2012. doi:10.1007/s10973-012-2395-2.
Lacerda LG, Carvalho Filho MAS, Demiate IM, Bannach G, Ionashiro M, Schnitzler E. Thermal behavior of corn starch granules under action of fungal a-amylase. J Therm Anal Calorim. 2008;93:445–9.
Colman TAD, Demiate IM, Schnitzler E. The effect of microwave radiation on some thermal, rheological and structural properties of cassava starch. J Therm Anal Calorim. 2012;. doi:10.1007/s10973-012-2866-5.
Winter CK, Davis S. Organic foods. J Food Sci. 2006;71:117–24.
Magkos F, Arvaniti F, Zampelas A. Organic food: buying more safety or juste peace of mind? A critical review of the literature. Crit Rev Food Sci Nutr. 2006;46:23–56.
Singh J, Kaur L, McCarthy OJ. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications: a review. Food Hydrocol. 2007;21:1–22.
Matsuguma LS, Lacerda LG, Schnitzler E, Carvalho-Filho MAS, Franco CML, Demiate IM. Characterization of native and oxidized starches of two varieties of Peruvian carrot (Acacia xanthorriza B.) from two production areas of Paraná state, Brazil. Braz Arch Biol Technol. 2009;52:701–13.
Garrido LH, Schnitzler E, Zortéa MEB, Thaís de Souza Rocha TS, Demiate IM. Physicochemical properties of cassava starch oxidized by sodium hypochlorite. J Food Sci Technol. 2012;. doi:10.1007/s13197-012-0794-9.
Beninca C, Colman TAD, Lacerda LG, Carvalho-Filho MAS, Demiate IM, Bannach G, Schnitzler E. Thermal, rheological, and structural behaviors of natural and modified cassava starch granules, with sodium hypochlorite solutions. J Therm Anal Calorim. 2012;. doi:10.1007/s10973-012-2592-z.
Kweon M, Haynes L, Slade L, Levine H. The effect of heat and moisture treatments on enzyme digestibility of AeWx, Aewx and aeWx corn starches. J Therm Anal Calorim. 2000;59:571–86.
Zavareze ER. Dias ARG. Impact of heat–moisture treatment and annealing in starches: a review. Carbohydr Polym. 2011;83:317–28.
Watcharatewinkul Y, Puttanlek C, Rungsardthong V, Uttapap D. Pasting properties of a heat–moisture treated canna starch in relation to its structural characteristics. Carbohydr Polym. 2009;75:505–11.
Chung H-J, Cho A, Lim S-T. Effect of heat–moisture treatment for utilization of germinated brown rice in wheat noodle. LWT Food Sci Technol. 2012;47:342–7.
Balasubramanian S, Sharma R, Kaur J, Bhardwaj N. Characterization of modified pearl millet (Pennisetum typhoides) starch. J Food Sci Technol. 2011;. doi:10.1007/s13197-011-0490-1.
Takahasi T, Miura M, Ohisa N, Mori K, Kobayashi S. Heat treatments of milled rice and properties of the flours. Cereal Chem. 2005;82:228–32.
Lorlowhkarn K, Naivikul O. Modification of rice flour by heat–moisture treatment (HMT) to produce rice noodles. Kasetsart J. 2006;40:135–43.
Shih F, King J, Daigle K, An H-J, Ali R. Physicochemical properties of rice starches modified by hydrothermal treatments. Cereal Chem. 2007;84:527–31.
Juszczak L, Fortuna T, Krok F. Non-contact atomic force microscopy of starch granules surface. Part I. Potato and tapioca starches. Starch/Stärke. 2008;55:1–7.
Juszczak L, Fortuna T, Krok F. Non-contact atomic force microscopy of starch granules surface. Part II. Selected cereal starches. Starch/Stärke. 2008;55:8–18.
Colman TAD, Bicudo SCW, Lacerda LG, Carvalho-Filho MAS, Demiate IM, Bannach G, Schnitzler E. Characterization of wheat starch by thermoanalytical, rheological and atomic force microscopy techniques. Braz J Therm Anal. 2012;1:62–5.
Zhang L, Xie W, Zhao X, Liu Y, Gao W. Study on the morphology, crystalline structure and thermal properties of yellow ginger starch acetates with different degrees of substitution. Thermochim Acta. 2009;495:57–62.
Zavareze ER, Storck CR, Castro LAS, Schirmer MA, Dias ARG. Effect of heat–moisture treatment on rice starch of varying amylose content. Food Chem. 2010;121:358–65.
Miao M, Zhang T, Jiang B. Characterisations of kabuli and desi chickpea starches cultivated in China. Food Chem. 2009;113:1025–32.
Falade KO, Kolawole TA. Effect of γ-irradiation on colour, functional and physicochemical properties of pearl millet [Pennisetum glaucum (L) R. Br.] cultivars. Food Bioprocess Technol. 2012;. doi:10.1007/s11947-012-0981-8.
Gunaratne A, Hoover R. Effect of heat–moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydr Polym. 2002;49:425–37.
Acknowledgements
The financial resources for this study were provided by the Fundação Araucária, PR, Brazil, the FINEP-Brazil, CAPES-Brazil and the CNPq-Brazil, whom the authors gratefully acknowledge.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrade, M.M.P., de Oliveira, C.S., Colman, T.A.D. et al. Effects of heat–moisture treatment on organic cassava starch. J Therm Anal Calorim 115, 2115–2122 (2014). https://doi.org/10.1007/s10973-013-3159-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3159-3