Abstract
For the first time, calorimetric studies performed at very low temperature highlighted the preliminary and required conditions for further aggregation/coacervation of peptides from elastin in solution through the structural water reorganization around the peptides. For this purpose, we firstly characterized by turbidimetry and differential scanning calorimetry the synthetic S4 fragment peptide containing the XGGZG motif (where X and Z correspond to Valine or Leucine) which is able to form amyloid fibres under certain conditions. We also investigated two medium-sized elastin-related model elastin peptides containing the VGVPG motif (E50 and E18) as well as their analogues where proline is hydroxylated in hydroxyproline. These peptides were shown to coacervate, and a close correlation was found between the inverse transition temperature obtained by turbidimetry and the clathrate-like structures evidenced at low temperature by the calorimetric method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface hydration is closely connected to the structural stability and flexibility of proteins, and its knowledge is essential for a better understanding of biological activities and pathologies. An important goal concerning elastin research is to explain its ability to extend and contract in repetitive motion in the hydrated state. Forty years of research on the dynamics of elastin are reviewed by Muiznieks et al. [1] emphasizing the crucial role of the structural disorder of both the peptide chain and the surrounding water in the mechanism of elasticity that can be well described by the entropy-driven model [2, 3]. Because of the extreme insolubility of elastin, most of the investigations are carried out on tropoelastin, its soluble precursor. The primary structure of tropoelastin consists of cross-linking regions—where the lysyl residues are located—alternating with large hydrophobic domains [4] that were shown to be responsible for elasticity [5]. Tamburro’s group synthesized a large series of polypeptides sequences encoded by the distinct exons of human tropoelastin and evidenced labile conformations by circular dichroism (CD) and nuclear magnetic resonance (NMR) depending on the experimental conditions and environment. These polypeptides alternate between different conformations in equilibrium, giving rise to a conformational ensemble that includes α-helices (favoured in structuring solvent like TFE), folded sequences β turns and β strands [6] and the more extended polyproline II (PPII) [7, 8]. PPII structures are defined by an absence of intramolecular and intermolecular hydrogen bonds and can, therefore, interconvert between conformations. Nevertheless, even if the great majority of these polypeptides sequences form organized structures, only a few of them are able to coacervate [9, 10] or to irreversibly aggregate into amyloid structure [11–13]. Coacervation, the initial step of fibrillogenesis in native tropoelastin [14], is an entropy-driven process due to the cooperative interactions between hydrophobic domains [15, 16]. Below the coacervation temperature, hydrophobic peptides are certainly shielded by clathrate-like water which prevents the self-aggregation. At the coacervation temperature, these clathrate-like structures would be disrupted, and the self-interaction of hydrophobic domain becomes possible [17]. Coacervation phenomenon and the associated inverse temperature transition are affected by the amino acid sequences [18], the residue hydrophobicity [9], the number of proline residues and their spacing in the elastin-like polypeptides [19], and by the introduction of electronegative groups [20]. Nevertheless, if these structural requirements are essential for the coacervation process [21], the coacervation temperature is also largely influenced by experimental parameters such as pH, solution concentration and NaCl content [22, 23]. Moreover, the hydroxylation of proline in (2S,4R)-4-hydroxyproline (Hyp) is one of the most frequent post-translational modification in the proteins of the extracellular matrix-like collagen and elastin. In elastin, the exact role of this modification is not yet understood, and a very recent work showed that the self-assembly of some elastin polypeptides was significantly altered by the presence of Hyp, evidencing different supramolecular structures [24].
If hydrophobic elastin sequences are in general optimized to avoid an amyloid fate, studies have evidenced the propensity of some elastin sequences to form amyloid fibres [25]. These authors suggested that the substitution of the rigid proline amino acids with the more flexible glycine residues accounts for this irreversible precipitation [26]. This precipitation depends on some parameters such as temperature, time and hydration level, and the main structures observed before aggregation are the β conformations which act as seeds for β sheet structures [13, 27]. To highlight the propensity of Glycine-rich motif to amyloid forming, Salvi et al. have extensively studied the influence of amino acid specificities or solvation on the molecular and supramolecular organization by CD, NMR, AFM and XPS [28, 29]. Elastin-like or amyloid-like peptides adopt complex supramolecular structures, showing either hydrophobic or hydrophilic surfaces. Molecular simulations of the monomeric and aggregated states strongly evidence that elastin-like and amyloid-like peptides have distinctive backbone hydration and peptide–peptide hydrogen bonding [26]. More generally, the self-assembly molecules domain is of rising interest, involving the construction of smart materials able to perform specific functions at the molecular scale. If the self-assembly of organic nanostructures is mainly driven by weak intermolecular forces (van der Waals forces, hydrogen bonding and hydrophobic effect), strong intermolecular interaction such as ionic covalent and coordination bonds can also play a central role in the formation of self-assembled materials, enhancing the dynamic properties of the final supramolecule [30].
Differential scanning calorimetry (DSC) has proven to be a useful technique to study phase transitions in protein mixtures [31], protein denaturation in biological compounds [32], aggregation and self-assembly of proteins and peptides [33, 34]; it is well suited for quantifying freezable water in hydrated elastin [35, 36], for revealing the physical structure of collagen and elastin in cardiovascular tissues [37] and for analyzing recurring sequences of elastin such as pentapeptide GLGGV [38], poly(GVGVP) [39] and the synthetic S4 fragment [40] in the condensed state. Finally, it also runs in solution, evidencing for example the hydrophobic hydration of poly(VPGVG) [41] or the thermal behaviour of exon 6 [42].
In this work, we propose to compare the thermal behaviour of water in the solutions of distinct peptides from elastin that either coacervate or form amyloid-like structures to gain insight into the role of water in the mechanism of aggregation.
Experimental
Polypeptide synthesis and purification
Five polypeptides (Table 1) were synthesized by solid-phase methodology using an automatic synthesizer APPLIED BIOSYSTEM model 431 A. Fmoc/DCC/HOBT chemistry was used starting from (0.25 mmol) Wang resin (Nova Biochem Laufelfingen, Switzerland) for S4, E50, E50H polypeptide syntheses. In the case of E18 and E18H polypeptides having a proline at the C-terminus, the amidated polypeptides were synthesized using (0.25 mmol) Rink-amide resin (Novabiochem, Laufelfingen, Switzerland) to avoid side reactions. The Fmoc-amino acids were purchased from Novabiochem (Laufelfingen, Switzerland) and from Inbios (Pozzuoli, Italy). The cleavage of the peptides from resin was achieved by an aqueous mixture of 95 % trifluoroacetic acid (TFA). The peptides were lyophilised and purified by reversed-phase high-performance liquid chromatography (HPLC). A binary gradient was used, and the solvents were H2O (0.1 % TFA) and CH3CN (0.1 % TFA). The purity of peptides was assessed by electrospray mass spectrometry.
Turbidimetry
Experiments were performed on a Cary 50 UV spectrophotometer equipped with a Peltier temperature controller using quartz cells of 1 cm path length and reported as turbidimetry on apparent absorbance (TAA). The solution temperature was increased from 10 to 90 °C with 2 °C every 5 min and then decreased back to 10 °C, monitoring the absorbance under stirring after 5 min to reach the temperature equilibrium. S4 peptides were dissolved in TBS buffer [Tris (50 mmol L−1), NaCl (1.5 mol L−1) and CaCl2 (1.0 mmol L−1) (pH 7.0)] to a concentration of 2 g L−1, and the absorbance was monitored at 440 nm. The E50, E50H, E18 and E18H peptides were dissolved in TBS buffer (pH 7.5) to a concentration of 100 g L−1, and the absorbance was monitored at 400 nm.
Differential scanning calorimetry (DSC)
The DSC curves were recorded with a Pyris Diamond calorimeter (PERKIN ELMER). The calorimeter was calibrated using cyclohexan and indium as standards. Solution samples (15 µL, corresponding to a sample mass of 15 mg) were sealed in hermetic aluminium pans, and an empty pan was used as reference. All the curves were recorded with a cooling or heating rate of 10 °C min−1. The concentration of S4 peptide was 30 g L−1 (~17 mmol L−1) in TBS buffer. The concentration of polypeptides E50, E50H, E18 and E18H was 100 g L−1 (~25 mmol L−1) in TBS buffer.
Results and discussion
TBS buffer
In order to determine the origin of the different transitions of the peptides/TBS buffer systems, a preliminary thermal characterization of the buffer alone was performed. The DSC cooling and heating curves of the TBS buffer in the −80/60 °C temperature range are presented in Fig. 1.
On the cooling scan are observed 2 exothermic peaks at −20 and −45 °C. As previously observed in saline solutions, these events are ascribed to the two-phase transitions of the TBS buffer [43], namely the freezing of primary ice (intense signal) and the freezing of the eutectic phase, respectively. On the heating scan are observed two endothermic transitions, namely the melting of the eutectic phase at −22 °C and the melting of primary ice at 0 °C [43]. It is noteworthy that the two-phase transitions recorded on cooling are shifted towards low temperature when compared with the transitions recorded on heating due to the supercooling of the liquids.
S4 in TBS buffer
In order to investigate the aggregation properties of the S4 peptide, turbidimetry experiments were carried out as a function of temperature (Fig. 2). The aggregation is experimentally measured by spectrophotometry as a sharp increase in the apparent absorbance value concomitant with heating to a critical temperature. These experiments are generally performed in TBS buffer, since the presence of ions makes the repulsive force between peptides charges decrease and promotes association, resulting in an increased tendency of the peptides to aggregate by agitation [44].
As previously reported [45], the turbidity measurements showed that S4 peptide undergoes an irreversible aggregation triggered by the temperature. The temperature at which the aggregation started was 60 °C and reached the highest value at 90 °C. After that, cooling the solution did not reduce the TAA. Combined with microscopy and conformational studies [40, 45], these findings strongly suggest for S4 an irreversible phase transition compatible with an amyloid-like behaviour.
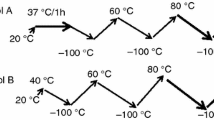
DSC Curves of S4 in TBS buffer (30 g L−1) were recorded in the cooling mode (Fig. 3) after a previous heating ramp up to the indicated temperature. On the whole, cooling scans are observed 3 exothermic peaks at −70, −50 and −20 °C. As previously shown, the events occurring at −20 and −50 °C originate from the TBS buffer.
As for the exothermic peak observed at −70 °C, undetectable in TBS buffer alone, it arises from the combination between solvent and S4 peptide. The low-temperature value of this transition is rather unusual when compared with literature data on the thermal characterization of hydrated biomolecules. It is also noteworthy that a very restricted number of DSC studies have been performed in this temperature range for biological systems, and none of the studies have been presented in this temperature range on such peptides/solution systems. Nevertheless, a quite similar thermal transition is detected for the crystallization of water adsorbed in clays [46] or activated carbon [47] in the −60/−50 °C temperature range. In the case of hydrated hydrophilic polymers, loosely bound water exhibits a crystallization phenomenon with considerable supercooling in the −50/−40 °C range [48]. In cellulose, the freezing of water bound to hydroxyl groups was shown to decrease with the structural organization of the polymer [49]. By analogy with these previous studies, the exothermic event recorded at −70 °C could be ascribed to the crystallization of freezing bound water in the hydration shell of S4 and could be interpreted in terms of hydrophilic hydration. It is noteworthy that this peculiar transition is in general better evidenced in the cooling scans than in the heating ones, where it can be completely absent because of its continuous character [46].
This peak increases when the solution is heated above 70 °C, decreases when the solution is heated at 80 °C and completely disappears when the sample is maintained at 80 °C during 3 h. As shown by turbidimetry experiments, S4 peptide in TBS solution self-aggregates into amyloid fibres with increasing temperature. Moreover, CD studies [45, 50] evidenced that the dominant conformation of S4 peptide in its initial state was the PPII helix, extended and flexible structure devoid of intramolecular hydrogen bonds, which interconverts into folded β-sheet conformation through hydrophobic interaction when the temperature increases, reaching the irreversible, cross-β amyloid-like structure above 80 °C. The hydrophilic hydration of S4 peptide can be thus associated with the PPII conformation; once formed, the cross-β structure is devoid of such a hydrophilic hydration, leading to the disappearance of the exothermic event at −70 °C in the DSC curves. As shown in Fig. 3, this hydrophilic hydration disappears after complete aggregation into amyloid fibrils that are characterized by an ordered, water-excluding core-containing extensive secondary structures [26]. Nevertheless, it was also evidenced by molecular simulations [26, 51] on (GVPGV)7 and (GVAGGV)6 that hydrophobic forces drive the emergence of collapsed water-swollen conformations of polypeptide chains which precede formation of the hydrophobic core; this fact could explain the reminiscent and increase of hydrophilic hydration in the curves corresponding to the prefibrillar states of S4 as shown in Fig. 3 (after a first heating up to 60 and 70 °C).
Influence of ageing on S4 solutions
We have superimposed the cooling curves of S4 in solution in TBS recorded in the low-temperature zone in the initial state and after storage at 4 °C for 15 and 30 days (Fig. 4).
The thermal signature of the hydrophilic hydration at −70 °C increases with storage of 15 days—which can considered as the optimal time to maximize the polypeptide chains/water hydrogen bonds—and slowly decreases with further ageing. These slow changes show that the hydration behaviour—connected to the self-assembly behaviour of the peptide—is able to vary with storage, in good agreement with the suggested multistate processes of protein hydration dynamics [52], and with the role of water observed in proteins [13]. The influence of the time and/or temperature on the structure is corroborated by the work of Salvi et al. showing by CD and FTIR investigations that preferential conformations can be affected by time of analyses or storage [29, 53] and pointing out by AFM experiments that the propensity of peptides to form amyloid fibres depends on the water activity in the medium.
E50, E50H, E18 and E18H polypeptides in TBS buffer
In order to correlate results from turbidimetry and calorimetry, the four elastin-like peptides were studied in TBS solution. We reported in Fig. 5 the turbidimetry experiments performed for E50 and E50H (heating mode). The same experiments (not shown) were performed for E18 and E18H.
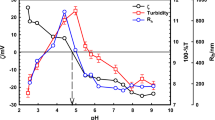
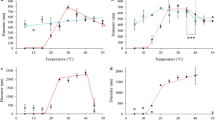
In this case, the sharp increase of the apparent absorbance was associated with a coacervation phenomenon [24] since the processus is reversible on cooling. The coacervation temperature was determined as the temperature value at which 50 % of coacervation took place. As expected, the coacervation temperatures of the peptides containing hydroxyproline are higher than the ones of the parent polypeptides containing the proline residues. In particular, E50 and E18 have coacervation temperatures of 35 and 50 °C, respectively. Conversely, E50H and E18H have coacervation temperatures of 60 and 85 °C, respectively. The introduction of the hydroxyl group on the pyrrolidine ring of proline reduces the hydrophobicity of the peptides, reducing propensity to coacervate.
The successive DSC heating scans of E50H polypeptides with different final temperatures are reported in Fig. 6. The first DSC scan performed between −100 and 80 °C is characterized by the two-phase transitions of the TBS solution at −22 and 0 °C as previously shown in Fig. 1. In the low-temperature range is detected a small exothermic event at −50 °C. Such a type of event has been already detected in the curves of synthetic polymers and associated with the cold crystallization phenomenon occurring in the heating mode. This mode is reversible on the second scan performed after the first heating up to 80 °C. Nevertheless, this mode is not detected on the third and fourth scans performed after the heating up to −30 °C. It is detected again on the fifth scan, recorded after the heating up to −10 °C associated with to the melting of the eutectic phase at −22 °C. A part of this peculiar ordered structure formed at −50 °C must disrupt at the melting of the eutectic phase, and in this way it is intimately connected with water. By analogy with previous DSC studies on CCl3F in a water-in-oil emulsion showing that clathrate compounds could be formed during DSC heating scans [54], we propose to associate this low-temperature exothermic event with the formation of clathrate-like forms of water at the vicinity of the hydrophobic residues of the polypeptidic chains. In these structures, water molecules are supposed to align themselves parallel to the non-polar side chains, interacting with each other rather than with protein atoms and would shield the hydrophobic residues from self-interactions [17]. Therefore, this exothermic event can be considered as the thermal answer of the hydrophobic hydration of the polypeptides E50 and E50H; such a type of hydration was previously estimated at 100 or more molecules of water per pentamers in hydrophobic sequences from tropoelastin [17].
The DSC heating scans of E50 and E50H polypeptides are reported in Fig. 7. Between −30 and 10 °C, DSC curves are characterized by the two-phase transitions of the TBS solution, namely the melting of the eutectic phase at −22 °C and the melting of primary ice at 0 °C [43]. It is noteworthy that the two-phase transitions recorded on heating are shifted towards high temperature when compared with the transitions recorded on cooling as done in Fig. 3; the shift towards low temperature during cooling is due to the supercooling of the liquids. At low temperature (enlarged zone of Fig. 7) is detected a small exothermic event at −75 and −50 °C for E50 and E50H, respectively. Such a type of event has been already detected in the curves of synthetic polymers and associated with the cold crystallization phenomenon occurring in the heating mode. Comparing the temperature of this exothermic event in E50 and E50H, it is patently obvious that hydroxylation of proline leads to a significant shift towards high temperature of the clathrate water formation. To confirm the effect of the proline hydroxylation on the clathrate water formation, similar DSC experiments have been performed on E18 and E18H, and the resulting curves are presented in Fig. 8. In the two successive scans performed for E18 and E18H, the exothermic peaks associated with clathrate-like water appeared at −73 °C and at −43 °C, respectively, with a good reproducibility indicative of the same kinetics behaviour. In a similar way as for E50 and E50H, hydroxylation of proline leads to a shift towards high temperature of the clathrate formation monitored by DSC. These DSC experiments confirm that the presence of the hydroxyl group of hydroxyproline in the polypeptidic chain, which can undergo hydrogen bonding with water molecules, alters the equilibrium among hydrophobic and hydrophilic hydration [24]. It was previously argued [17] that hydrogen bonds between clathrate water molecules are disrupted when the temperature increases, exposing the hydrophobic domains for self-interaction and thus leading to coacervation phenomenon. In order to correlate the clathrate water behaviour with the coacervation phenomenon, we plotted in Fig. 9 the temperatures of clathrate formation and coacervation for the four peptides. As shown in Fig. 9, the gap between the cold crystallization temperatures of E50/E50H and E18/E18H from DSC experiments is exactly equal to the gap between the coacervation temperatures from turbidimetry experiments, confirming the close relationship between these two thermally induced events. According to Cirulis and Keeley [55], we can wonder if the conformational changes in the polypeptide sequence are the cause or the result of the change in hydration. We can assume that the clathrate-like water whose formation is monitored by DSC in the solid state remains stable in the liquid state until the coacervation temperature, keeping the peptide unfolded [22]. At the coacervation temperature, the clathrate water molecules become as disordered as the surrounding bulk ones, and the hydrophobic residues are submitted to Van der Waals interactions, leading to the folded conformation. The presence of hydroxyproline in the polypeptidic chains alters the clathrate formation, enhancing hydrophilic hydration and leading to less-aligned water molecules around the hydrophobic residues. As a consequence, it induces a reducing propensity of the polypeptidic chain to coacervate: a higher temperature is required to induce coacervation, because a higher energy is required to destabilize the hydrophilic hydration shell. Moreover, a previous study showed that the reduced hydrophobicity of hydroxyproline alters the ultrastructure of aggregates, which show a more polymorphic aggregation pattern when compared with the fibrillar patter of proline-containing polypeptides. The long-range order of clathrate-like water seems to be a prerequisite for the further fibre formations.
Conclusions
This work shows that DSC can evidence distinct low-temperature behaviours for different elastin-like peptides solutions. On the one hand, the amyloidogenic S4 peptides possess in pure water a great level of disorder. In the presence of salts, this disordered phase leads to a hydrophilic hydration detectable by DSC. Once aggregated into their amyloid form, these S4 peptides do not exhibit either amorphous phase or hydrophilic hydration, confirming the ordered character of the water-excluding cross-β structure.
On the other hand, a hydrophobic hydration is revealed for the series of peptides that coacervate, confirming that elastin self-assembly is mainly triggered by hydrophobic interactions. The temperature of the clathrate-like water formation is closely connected to the amino acid sequence, with an important influence of proline hydroxylation, and can be used to plan the ability of the peptide to coacervate.
References
Muiznieks LD, Weiss AS, Keeley FW. Structural disorder and dynamics of elastin. Biochem Cell Biol. 2010;88:239–50.
Debelle L, Tamburro AM. Elastin: molecular description and function. Int J Biochem Cell Biol. 1999;31:261–72.
Urry DW, Hugel T, Seitz M, Gaub HE, Sheiba L, Dea J, et al. Elastin: a representative ideal protein elastomer. Philos Trans R Soc Lond B Biol Sci. 2002;357:169–84.
Wise SG, Weiss AS. Tropoelastin. Int J Biochem Cell Biol. 2009;41:494–7.
Tamburro AM, Bochicchio B, Pepe A. Dissection of human tropoelastin : exon-by-exon chemical synthesis and related conformational studies. Biochemistry. 2003;42:13347–62.
Bisaccia F, Castiglione-Morelli MA, Spisani S, Ostuni A, Serafini-Fracassini A, Bavoso A, et al. The amino acid sequence coded by the rarely expressed exon 26A of human elastin contains a stable beta-turn with chemotactic activity for monocytes. Biochemistry. 1998;37:11128–35.
Tamburro AM, Pepe A, Bochicchio B. Localizing alpha-helices in human tropoelastin: assembly of the elastin “puzzle”. Biochemistry. 2006;45:9518–30.
Bochicchio B, Floquet N, Pepe A, Alix AJP, Tamburro AM. Dissection of human tropoelastin: solution structure, dynamics and self-assembly of the exon 5 peptide. Chemistry. 2004;10:3166–76.
Urry DW, Luan CH, Parker TM, Gowda D, Prasad K, Reid MC, et al. Temperature of polypeptide inverse temperature depends on mean residue hydrophobicity. J Am Chem Soc. 1991;113:4346–8.
Reiersen H, Clarke AR, Rees A. Short elastin-like peptides exhibit the same temperature-induced structural transitions as elastin polymers: implications for protein engineering. J Mol Biol. 1998;283:255–64.
Pepe A, Guerra D, Bochicchio B, Quaglino D, Gheduzzi D, Pasquali Ronchetti I, et al. Dissection of human tropoelastin: supramolecular organization of polypeptide sequences coded by particular exons. Matrix Biol. 2005;24:96–109.
Ostuni A, Bochicchio B, Armentano MF, Bisaccia F, Tamburro AM. Molecular and supramolecular structural studies on human tropoelastin sequences. Biophys J. 2007;93:3640–51.
Bochicchio B, Pepe A, Flamia R, Lorusso M, Tamburro AM. Investigating the amyloidogenic nanostructured sequences of elastin: sequence encoded by exon 28 of human tropoelastin gene. Biomacromolecules. 2007;8:3478–86.
Wise SG, Yeo GC, Hiob MA, Rnjak-Kovacina J, Kaplan DL, Ng MKC, et al. Tropoelastin: a versatile, bioactive assembly module. Acta Biomater. 2014;10:1532–41.
Luan CH, Parker TM, Prasad KU, Urry DW. Differential scanning calorimetry studies of NaCl effect on the inverse temperature transition of some elastin-based. Biopolymers. 1991;31:465–75.
Luan CH, Parker TM, Gowda DC, Urry DW. Hydrophobicity of amino acid residues: differential scanning calorimetry and synthesis of the aromatic analogues of the polypentapeptide of elastin. Biopolymers. 1992;32:1251–61.
Yeo GC, Keeley FW, Weiss AS. Coacervation of tropoelastin. Adv Colloid Interface Sci. 2011;167:94–103.
Ribeiro A, Arias FJ, Reguera J, Alonso M, Rodríguez-Cabello JC. Influence of the amino-acid sequence on the inverse temperature transition of elastin-like polymers. Biophys J. 2009;97:312–20.
Muiznieks LD, Keeley FW. Proline periodicity modulates the self-assembly properties of elastin-like polypeptides. J Biol Chem. 2010;285:39779–89.
Pepe A, Crudele MA, Bochicchio B. Effect of proline analogues on the conformation of elastin peptides. New J Chem. 2013;37:1326.
Maeda I, Fukumoto Y, Nose T, Shimohigashi Y, Nezu T, Terada Y, et al. Structural requirements essential for elastin coacervation: favorable spatial arrangements of valine ridges on the three-dimensional structure of elastin-derived polypeptide (VPGVG)n. J Pept Sci. 2011;17:735–43.
Vrhovski B, Jensen S, Weiss AS. Coacervation characteristics of recombinant human tropoelastin. Eur J Biochem. 1997;250:92–8.
Reguera J, Urry DW, Parker TM, McPherson DT, Rodríguez-Cabello JC. Effect of NaCl on the exothermic and endothermic components of the inverse temperature transition of a model elastin-like polymer. Biomacromolecules. 2007;8:354–8.
Bochicchio B, Laurita A, Heinz A, Schmelzer CEH, Pepe A. Investigating the role of (2S,4R)-4-hydroxyproline in elastin model peptides. Biomacromolecules. 2013;14:4278–88.
Miao M, Bellingham CM, Stahl RJ, Sitarz EE, Lane CJ, Keeley FW. Sequence and structure determinants for the self-aggregation of recombinant polypeptides modeled after human elastin. J Biol Chem. 2003;278:48553–62.
Rauscher S, Baud S, Miao M, Keeley FW, Pomès R. Proline and glycine control protein self-organization into elastomeric or amyloid fibrils. Structure. 2006;14:1667–76.
Pepe A, Armenante MR, Bochicchio B, Tamburro AM. Formation of nanostructures by self-assembly of an elastin peptide. Soft Matter. 2009;5:104.
Salvi AM, Moscarelli P, Satriano G, Bochicchio B, Castle JE, Lucano A, et al. Influence of amino acid specificities on the molecular and supramolecular organization of glycine-rich elastin-like polypeptides in water. Biopolymers. 2011;95:702–21.
Salvi AM, Moscarelli P, Bochicchio B, Lanza G, Castle JE. Combined effects of solvation and aggregation propensity on the final supramolecular structures adopted by hydrophobic, glycine-rich, elastin-like polypeptides. Biopolymers. 2013;99:292–313.
Dhotel A, Chen Z, Delbreilh L, Youssef B, Saiter J-M, Tan L. Molecular motions in functional self-assembled nanostructures. Int J Mol Sci. 2013;14:2303–33.
Ostrowska-Ligęza E, Górska A, Wirkowska M, Koczoń P. An assessment of various powdered baby formulas by conventional methods (DSC) or FT-IR spectroscopy. J Therm Anal Calorim. 2012;110:465–71.
Briere L-AK, Brandt J-M, Medley JB. Measurement of protein denaturation in human synovial fluid and its analogs using differential scanning calorimetry. J Therm Anal Calorim. 2010;102:99–106.
Suh Y, Kim BJ, Tam KC, Aucoin MG. Detection and characterization of hemoglobin dissociation and aggregation using microcalorimetry. J Therm Anal Calorim. 2013;115:2159–69.
Tiné MR, Alderighi M, Duce C, Ghezzi L, Solaro R. Effect of temperature on self-assembly of an ionic tetrapeptide. J Therm Anal Calorim. 2010;103:75–80.
Samouillan V, André C, Dandurand J, Lacabanne C. Effect of water on the molecular mobility of elastin. Biomacromolecules [Internet]. 2004;5:958–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15132687.
Samouillan V, Tintar D, Lacabanne C. Hydrated elastin: dynamics of water and protein followed by dielectric spectroscopies. Chem Phys. 2011;385:19–26.
Samouillan V, Dandurand J, Lacabanne C, Stella A, Gargiulo M, Degani A, et al. Analysis of the molecular mobility of collagen and elastin in safe, atheromatous and aneurysmal aortas. Pathol Biol (Paris). 2012;60:58–65.
Megret C, Guantieri V, Lamure A, Pieraggi MT, Lacabanne C, Tamburro AM. Phase transitions and chain dynamics, in the solid state, of a pentapeptide sequence of elastins. Int J Biol Macromol. 1992;14:45–9.
Rodriguez-cabello JC, Alonso M, Diez MI, Caballero MI, Herguedas MM. Structural investigation of the poly(pentapeptide) of elastin, poly(GVGVP), in the solid state. Macromol Chem Phys. 1999;200:1831–8.
Dandurand J, Samouillan V, Lacoste-Ferre MH, Lacabanne C, Bochicchio B, Pepe A. Conformational and thermal characterization of a synthetic peptidic fragment inspired from human tropoelastin: signature of the amyloid fibers. Pathol Biol (Paris). 2014;60:100–7.
Rodrıguez-Cabello JC, Alonso M, Perez T, Mar Herguedas M. Differential scanning calorimetry study of the hydrophobic hydration of the elastin-based polypentapeptide poly(VPGVG), from deficiency to excess of water. Biopolymers. 2000;54:282–8.
Tintar D, Samouillan V, Dandurand J, Lacabanne C, Pepe a, Bochicchio B, et al. Human tropoelastin sequence: dynamics of polypeptide coded by exon 6 in solution. Biopolymers. 2009;91:943–52.
Kamasa P, Bokor M, Pyda M, Tompa K. DSC approach for the investigation of mobile water fractions in aqueous solutions of NaCl and Tris buffer. Thermochim Acta [Internet]. 2007 [cited 2014 Jan 17];464:29–34.
Sasahara K, Yagi H, Naiki H, Goto Y. Thermal response with exothermic effects of beta2-microglobulin amyloid fibrils and fibrillation. J Mol Biol. 2009;389:584–94.
Bochicchio B, Pepe A, Delaunay F, Lorusso M, Baud S, Dauchez M. Amyloidogenesis of proteolytic fragments of human elastin. RSC Adv. 2013;3:13273.
Kozlowski T. Low temperature exothermic effects on cooling of homoionic clays. Cold Reg Sci Technol. 2011;68:139–49.
Gun’ko VM, Turov VV, Zarko VI, Goncharuk OV, Nychiporuk YM, Kozynchenko OP, et al. Interfacial behavior of polar, weakly polar, and nonpolar compounds bound to activated carbons. J Colloid Interface Sci. 2013;404:140–9.
Hatakeyama H, Hatakeyama T. Interaction between water and hydrophilic polymers. Thermochim Acta. 1998;308:3–22.
Nakamura K, Hatakeyama T, Hatakeyama H. Studies on bound water of cellulose by differential scanning calorimetry. Text Res J. 1981;51:607–13.
Bochicchio B, Tamburro AM. Polyproline II structure in proteins: identification by chiroptical spectroscopies, stability, and functions. Chirality. 2002;14:782–92.
DuBay KF, Pawar AP, Chiti F, Zurdo J, Dobson CM, Vendruscolo M. Prediction of the absolute aggregation rates of amyloidogenic polypeptide chains. J Mol Biol. 2004;341:1317–26.
Halle B. Protein hydration dynamics in solution: a critical survey. Philos Trans R Soc Lond B Biol Sci. 2004;359:1207–23 discussion 1223–4, 1323–8.
Flamia R, Zhdan PA, Martino M, Castle JE, Tamburro AM. AFM study of the elastin-like biopolymer poly(ValGlyGlyValGly). Biomacromolecules. 2004;5:1511–8.
Fouconnier B, Komunjer L, Ollivon M, Lesieur P, Keller G, Clausse D. Study of CCl3F hydrate formation and dissociation in W/O emulsion by differential scanning calorimetry and X-ray diffraction. Fluid Phase Equilib. 2006;250:76–82.
Cirulis JT, Keeley FW. Kinetics and morphology of self-assembly of an elastin-like polypeptide based on the alternating domain arrangement of human tropoelastin. Biochemistry. 2010;49:5726–33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dandurand, J., Samouillan, V., Lacabanne, C. et al. Water structure and elastin-like peptide aggregation. J Therm Anal Calorim 120, 419–426 (2015). https://doi.org/10.1007/s10973-014-4254-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4254-9