Abstract
In this work, nanocomposites of ABS/PC blends reinforced with organically modified montmorillonite (OMMT) were prepared by melt blending in a twin screw extruder, and an assessment of the thermal degradation mechanisms was performed by thermogravimetric analysis. The incorporation of PC improves the thermal resistance of ABS/PC blends, with respect to pure ABS. The addition of OMMT alters the degradation mechanism and modifies the properties of blends with higher PC content, where an increase of the degradation temperature corresponding to PC becomes obvious, in comparison with the respective unreinforced blends. The gross calorific value was calculated using an oxygen bomb calorimeter, and in most of the examined nanocomposites, an inverse trend was observed between this property and the residue calculated after thermogravimetric analysis in inert atmosphere. Based on the above results, the thermal degradation behavior of ABS/PC nanocomposites was interpreted by the heat barrier effect, caused via the formation of a carbonaceous silicate char, which insulates the underlying material creating a protective barrier to heat and mass transfer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As environmental regulations have restricted the use of halogen-containing flame retardants (FR) because of the hazards of generating toxic gases and high levels of smoke, there is an increasing interest to halogen-free FR compounds. Phosphorus-containing additives, such as triphenylphosphate (TPP), resorcinol-bis(diphenyl phosphate) (RDP), and bisphenol A bis(diphenyl phosphate) (BDP), are among the non-halogenated FR [1, 2]. It also has been shown that organoclay fillers may be utilized as alternative flame-retardant additives for polymers [3].
Several mechanisms have been proposed to describe the flame-retardant properties of polymer/clay nanocomposites [4]. Gilman et al. [5, 6] and Qin et al. [4], who have studied clay reinforcements, attributed the improvement in flammability properties of PA6/clay, PS/clay, and PP/clay nanocomposites to a condensed-phase flame-retardant mechanism and not to a gas effect. According to Qin et al. [4], the flame retardancy of PP/clay nanocomposites is associated with two decisive factors: one is the thermal-oxidative degradation of the matrix and the other is the diffusion of volatile decomposed products and heat transfer in the condensed phase. The thermal-oxidative degradation plays an important role in the combustion of PP/clay nanocomposite [4].
On the basis of the fact that clays have good thermal stability, it is believed that the incorporation of inorganic components into organic polymers can improve their thermal stability, due to the heat insulator effect of the clay layers. Moreover, montmorillonite (MMT) layers are thought to reduce heat conduction. In the presence of nanodispersed MMT layers strongly interacting with polymer matrix, the motion of polymer chains is limited. This effect ensures additional stabilization in the case of polymer/MMT nanocomposites [7]. It is also considered that nanodispersed clay platelets act as insulators; moreover, the formation of tortuous path between layers further inhibits the passage of oxygen and volatile degradation products, thus enhancing the thermal stability of clay-containing composites (labyrinth effect) [7, 8].
The general view of the flame retardancy mechanism is that a carbonaceous silicate char builds up on the surface during burning, which creates a protective barrier to heat and mass transfer [4, 7, 8]. Generally, char formation during polymer degradation is a complex process; it occurs in several steps, which include the formation of conjugated double bond, cyclization, aromatization, fusion of aromatic rings, turbostratic char formation and finally graphitization [7]. Gilman et al. [6] studied the flame-retardant mechanism of PP/clay and PS/clay nanocomposites and reported that a high-performance carbonaceous silicate char builds up on the surface during burning; this insulates the underlying material and retards the mass loss rate of decomposition products. In another work [5], it was observed that until the char layer formation, the mass loss rate of PA6 and PA6/clay nanocomposite is the same, indicating that PA6/clay nanocomposite does not have greater inherent thermal stability than the pure PA6. This means that the char formation controls the mass loss rate and, therefore, the flammability. This is in agreement with TG data for PA6/clay nanocomposite, which show no increase in thermal stability. This residue layer is formed as the polymer burns away and the silicate layers reassemble into the multilayer char [6]. According to Lomakin et al. [9], collective action of chemical crosslinking and catalytic dehydration promoted by MMT presents necessary and sufficient condition of solid state carbonization reactions in the process of thermal-oxidative degradation and combustion of PE–MAPE–MMT nanocomposites.
The delaminated hybrid structure of PA6/clay nanocomposite appears to collapse during combustion [5]. Gilman et al. [6] and Zanetti et al. [8] found that the montmorillonite must be nanodispersed in order to affect the flammability. However, the clay need not to be completely delaminated [6]. Gilman [5] observed that the intercalated polyimide nanocomposite is more stable than the delaminated polyimide nanocomposite.
The accumulation of layered silicates on the burning/gasifying sample surface is considered to be due to two possible modes. One is that the layered silicates left on the sample surface, as the result of the decomposition of the polymer matrix by pyrolysis [4]. The aggregation of MMT layers in order to form stacks is attributed to the degradation of the organic treatment on the clay surface, which makes them more hydrophilic and less compatible with the resin [7]. The other mode is that the transportation of the layered silicates was pushed by numerous rising bubbles of degradation products and the associated convection flow in the melt from the interior of the sample toward the surface [4]. According to Ma et al. [10], the volatile combustible products formed from pyrolysis of ABS or ABS-g-MAH resin tend to leave the sample instantaneously. By leaving the polymer, these products cause the silicate to migrate to the sample surface. If the clay layers are well dispersed within the polymer and it concentrates on the surface upon heating, one would expect to see that the concentration of oxygen increasing and that of carbon decreasing. This result can be explained as follows: The polymer is lost by thermal degradation, and the clay becomes the dominant material at the surface and as (at temperature of 200–250 °C) the montmorillonite begins to decompose into its components, SiO2 and Al2O3·SiO2 with much lower surface energy than Al2O3.
The heat barrier effect could also provide superheated conditions inside the polymer melt, leading to extensive random scission of polymer chain and to the evolution of numerous chemical species which, trapped between clay layers, are likely to undergo secondary reactions. As a result, some degradation pathways could be promoted leading to enhanced charring. It is also suggested that the effect of more effective char production during thermal decomposition of polymer–clay nanocomposites may be derived from a chemical interaction between the polymer matrix and the clay layer surface during thermal degradation [7]. Studies on HDPE/organoclay nanocomposites by Barbarosa et al. [11] revealed that the increase in ammonium salt content of organoclay and the presence of polar compatibilizer favored the dispersion of clay in the polymer matrix, improving the thermal stability and reducing the flammability of the matrix. Similar behavior was reported by Kannan et al. [12] with thermoplastic polyurethane (TPU)/PP systems, when MA-g-PP compatibilizer and nanoclays were added.
The variable efficiency of MMT in improving the thermal stability of polymers is considered in terms of the complexity of degradation pathways or a result of radical stability. When there are more than one degradation pathways, as in the case of PS where both monomer and oligomer are produced, the presence of the clay can promote one degradation pathway at the expense of another. If the favored pathway leads to higher molecular weight material, then the polymer is degraded more slowly than it would be in the absence of clay. However, if there is only a single degradation pathway (or more probable ways theoretically but leading to the production of the same products, as for instance in the case PMMA), the clay cannot promote an evolution of different degradation products. Regarding the radical stability, if the radical species produced during the thermal decomposition of polymer are highly stable, i.e., they exhibit longer lifetimes, the probability that they will undergo secondary intermolecular reactions, especially radical recombination reactions, is also high—the role of the clay is then to prevent mass transport from the bulk and to permit radical recombination reactions, exerting thus a stabilization effect in the polymer/layered silicate nanocomposite [7].
Zhu et al. [13] assumed that structural iron must be the important site of radical trapping. Iron may be present in the clay either as an impurity or as a substitute for the aluminum or silicon atoms, participating in the structure of the aluminosilicate material. When iron is present as impurity, it cannot be well dispersed but must be clustered in particular regions of the structure, and thus it cannot be effective as a radical trap. On the other hand, if the iron is substituted within the clay structure, it must be nanodispersed within the polymer and, thus, can be available throughout the material. The above authors found that the presence of structural iron in clay does not lead to enhanced char formation, but affects the onset of degradation in an inert atmosphere and decreases the heat release rate.
Qin et al. [4] showed that the dispersion state of clay particles and their barrier effect have a minor effect on the thermal-oxidative stability and flammability of polymer matrix. On the contrary, the viscosity of PP/clay composites is far higher than pure PP during combustion. This suggests that there exists a physical crosslinked structure composed of clay particles and polymer chains. Moreover, the thermal degradation of alkylammonium salts in clay galleries can take place through the Hoffman mechanism, leading to volatilization of ammonia and the corresponding olefin:
The acidic sites are thus created on the silicate layers during heating. All these catalytic active sites can accept single electrons from donor molecules and form free radicals. On the one hand, these active sites catalyze the initial decomposition of polymer matrix. That is why, in addition to the release of the thermal degradation products of the organic treatment of the clay catalyzed by these active sites [4, 5, 8], the ignition time is shorter and the initial heat release rate (HRR) is higher for polymer/clay nanocomposite. On the other hand, the active sites can catalyze the formation of a protective coat-like char on the nanocomposite. The active sites can catalyze the dehydrogenation and crosslinking of polymer chains. Moreover, according to Zanetti et al. [8], the large surface area for clay–polymer contact in the nanocomposite favors the catalyzed dehydrogenation of the polymer to a conjugated polyene, which prevents volatilization to combustible volatiles by aromatization and charring. Protonic sites, formed on the silicate layers by Hoffman decomposition of the organic modifier of the clay, might enhance the catalytic action. This physical and chemical crosslinking can increase the thermal-oxidative stability, delay the thermal decomposition of polymer matrix, and decrease the HRR [4].
In the present work, nanocomposites of ABS/PC blends reinforced with OMMT were prepared by melt blending in a twin screw extruder, and an assessment of the thermal degradation mechanisms was performed by thermogravimetric (TG) analysis. The gross calorific value was evaluated and related with the TG results in order to better elucidate the thermal degradation mechanism. The aim of this study was to compare the obtained values of the above characteristic with the residue calculated after thermogravimetric analysis in inert atmosphere. The comparative investigation of these data can elucidate the mechanism of the thermal degradation of ABS/PC nanocomposites and, more specifically, suggest the existence of barrier effects that might control the material’s degradation.
Experimental
Materials
The terpolymer poly(acrylonitrile–butadiene–styrene), under the trade name Terluran® GP-35, was supplied by BASF SE. According to Galvan et al. [14], this type of ABS consists of 44 % acrylonitrile, 42 % styrene, and 14 % butadiene. The polycarbonate used in this work was Makrolon® 2805 (Bayer Materials Science AG). Commercial montmorillonite clay, under the trade name Cloisite® 30B, supplied by Rockwood Clay Additives GmbH, was also used as reinforcing nanofiller.
Preparation of nanocomposites
ABS and ABS/PC blends with consistencies, 70/30, 50/50, and 30/70 w/w, were prepared by melt mixing, in a co-rotating twin screw extruder, with L/D = 25 and 16 mm diameter (Haake PTW 16). The rotational speed of the extruder screws was 200 rpm. The extruder was heated at five zones along the cylinder and the die, according to the temperature profile presented in Table 1. During melt mixing in the twin screw extruder, no signs of essential thermomechanical degradation have been observed, at least by recording the torque versus extrusion time that remained constant. This stability of the polymeric melt must be attributed to the short time of residence into the extruder and the minimal exposure to the combined action of heat and shear.
Before mixing, ABS and Cloisite 30B were dried in a vacuum oven at 80 °C for 4 h, whereas PC was dried at 90 °C, overnight. After melt mixing, the obtained material in the form of a continuous strand was granulated in a Brabender pelletizer.
Characterization
X-Ray diffractometer (XRD)
The dispersibility of silicate layers into the ABS and the blends of ABS/PC was evaluated in a Siemens 5000 apparatus X-ray diffractometer (40 kV, 30 mA) using Cu Kα irradiation with a wavelength of λ = 0.154 nm. The diffractograms were scanned in the 2θ range from 2° to 10° with rate of 2° min−1. Samples for X-ray diffraction analysis were obtained from compression-molded plaques in order to avoid any preferred orientation of the clay.
Thermogravimetric analysis
TG measurements were performed in a Mettler Toledo (TG–DTA model) thermal gravimetric analyzer. The tests were run with samples of 8–10 mg from 25 to 800 °C, at a heating rate of 10 °C min−1, under nitrogen atmosphere.
Scanning electron microscopy (SEM)
SEM micrographs were obtained using a JEOL scanning electron microscope. The cryogenically fractured surfaces were immersed into an aqueous solution of NaOH (30 % w/v) at a temperature of 100 °C. In this way, the PC was hydrolyzed leaving intact the ABS domains. The etched samples were gold coated.
Oxygen bomb calorimeter
The gross calorific value (GCV) was calculated according to ASTM D5865, using a Parr 6400 oxygen bomb calorimeter.
Combustion thermochemistry
The theoretical estimation of gross calorific value for ABS and PC was performed. In case of ABS, the additive rule was applied, and for this reason, firstly, the gross calorific values of polyacrylonitrile, polybutadiene, and polystyrene were calculated.
Based on oxygen consumption during complete combustion [15]
Heats of combustion calculated from the oxygen consumption rely on the observation that a wide range of organic compounds, including polymers, have approximately the same heat of complete combustion per gram of diatomic oxygen consumed, E, which is equal to 13.1 kJ g−1 O2.
The net heat of complete combustion, Δh c, of a polymer is simply calculated if the atomic composition of the polymer is known, so that the balanced thermochemical reaction can be written as
In case of PC, the balanced chemical equation for complete combustion is
In case of ABS, which is a copolymer, the balanced chemical equations for complete combustion of polyacrylonitrile, polybutadiene, and polystyrene are correspondingly
So, the net heat of complete combustion, Δh c, of a polymer is estimated by the equation
where \( n{\text{o}}_{2} \) is the number of moles of O2 consumed in the balanced thermochemical equation, \( M{\text{o}}_{2} \) = 32 g mol−1 is the molecular weight of diatomic oxygen, and n p and M p are the number of moles and molecular weight of the polymer repeat unit.
The net heat of combustion is the gross heat of combustion minus the latent heat of vaporization of the water produced during the reaction. The gross calorific value is
where w H is the weight fraction of hydrogen in the sample.
Based on molar group additivity of heats of formation [15, 16]
Calculation of the heat of the combustion reaction of polymers was carried out using the principle of molar additivity of the heats of formation of the combustion products and reactants. The concept derives from the fact that enthalpy (H) is a state function, and therefore its change in any process is independent of the path from reactants to products. Thus, the overall enthalpy of a reaction is simply the sum of the enthalpies of the component reactions. In practice, the heat of combustion of the reaction can be calculated by subtracting the heat of formation of the products from the heat of formation of the reactants
where p and r denote products and reactants, respectively; Δh c is the net heat of complete combustion of the sample; and n is the number of moles of the molecule or polymer repeat unit.
The heats of formation of the products are
and reactants
For polymeric reactants, the molar heat of formation, ΔH f, can be estimated from the molar contributions of the chemical groups, which constitute the monomer or repeat unit. For the investigated polymers, the group contributions to the molar heat of formation of repeat unit (ΔΗ f) were calculated at T = 298 K.
Based on the stoichiometry of complete combustion reaction (2):
the molar heat of combustion of the examined material is
The gross heat of combustion per unit mass of the examined material is then
where M p is the molecular weight of the polymer repeat unit.
Based on molar group contributions [17]
The molar heat of complete combustion ΔH c is a thermodynamic property and should be calculable from the molar group contributions of the structural components. Assume that there is a molar heat of combustion, H, with units of kJ mol−1, in which each chemical group i in the polymer contributes according to its mole fraction, n i, in the repeat unit,
with H i, the molar heat of combustion of component i. Since
where N i and M i are the number of moles and molar mass, respectively, of group i in the polymer having a repeat unit of molar mass M, the heat of combustion can be expressed on a mass basis.
Based on Meraz equation [18]
Meraz et al. [18] introduce a new equation, based on thermochemical concepts, to calculate gross calorific value (GCV) from elemental composition. This equation is expressed in terms of mass percentages on a dry basis of carbon (%C), hydrogen (%H), oxygen (%O), nitrogen (%N), and sulfur (%S). The equation is
The mass percentages of elements in the examined materials are equal to their mass percentage in their repeat unit. Also, it is assumed that the examined materials did not have moisture or impurities. This equation neglects the inorganic carbon; hence, it is not very adequate when there is a significant concentration of it.
Results and discussion
X-ray diffraction (XRD)
From Fig. 1, the characteristic peak (001) of Cloisite 30B is observed at about 2θ = 5.05°, corresponding to an interlayer spacing of 1.75 nm. The XRD patterns of Cloisite 30B/ABS/PC blends in Fig. 1 show that the peak corresponding to the (001) plane reflection of clay shifts to lower angles and its intensity decreases. The increase of d-spacing of Cloisite 30B/ABS/PC nanocomposites suggests that some polymer chains penetrated the clay galleries forming an intercalated structure. The penetration of PC chains between the clay platelets is motivated by the formation of H-bonds among the OH-groups of organic modification of Cloisite 30B and carbonyl groups of PC [19], whereas the intercalation of ABS chains between the filler layers is induced by the interactions of OH-groups of organic modification and nitrile groups of ABS [20, 21]. It can also be seen that the secondary peak that corresponds to the (002) plane of nanocomposites shifted to higher angles. The (002) peak suggests that intercalated structures of clay are obtained in the nanocomposites. Similar findings were reported by Hong et al. [22] who studied Cloisite 10A/(70/30 w/w) ABS/PC blend at 3 and 5 phr, whereas Zong et al. [23] obtained intercalated structures for (5 mass%) OMMT/(3/2) PC/ABS composites. From Fig. 1, it can be observed that higher PC concentrations in ABS/PC blends seem to enhance the intercalation process in the prepared nanocomposites, as the 2θ values decrease. Interestingly, Wang et al. [24] reported that the increase of ABS content in PC/ABS nanocomposites does not produce any changes of the d 001 peaks.
Thermοgravimetric analysis
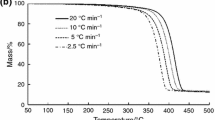
Effect of incorporation of PC to ABS
The decomposition temperature range of ABS is about 300–450 °C, while PC has a higher thermal stability and decomposes in the range of 500–600 °C, as shown in Fig. 2. From the derivative of weight change versus temperature in the examined blends, presented in Fig. 3, it is observed that the incorporation of 70 % PC in ABS decreases the height and increases the width of the corresponding peak. This was an expected effect because, as shown in Tables 2, 3 and 4, PC displays better thermal stability than ABS, so ABS/PC blends present improved thermal characteristics in comparison with pure ABS. The improvement of the thermal properties of ABS/PC blends is more evident as the PC content increases.
Nigam et al. [25] came at the same conclusion, as they observed that the onset degradation temperature and residue increase, as the PC content in ABS/PC blends increases. However, this effect does not seem to obey the “rule of mixtures,” probably due to the poor miscibility of the components of the prepared blends.
Balart et al. [26] prepared ABS/PC blends from waste of electric and electronic equipment (WEEE) at 100/0, 80/20, 20/80, and 0/100 and concluded that even with the addition of small amounts of ABS, the degradation curves are far from that of pure PC, but similar to those of pure ABS.
Effect of clay’s loading
The incorporation of nanofillers into pure ABS, as well as in 70/30 w/w ABS/PC blend, does not essentially affect their onset temperature of thermal degradation, T onset, for clay loadings up to 2 phr. However, at clay content 3 phr, a decrease of the T onset takes place (Table 2), probably due to the lower thermal stability of organoclay, caused by the organic modification. For the high PC content blends (50/50 and 30/70 w/w ABS/PC compositions), the onset of thermal degradation shifts to higher temperatures at low clay loadings, and then a decrease is observed.
The addition of 1 and 2 phr clay loadings in ABS increases slightly the maximum reaction rate temperature (T max) (Table 3), which is then reduced as the concentration of clay further increases to 3 phr. Regarding the examined ABS/PC blends, the incorporation of Cloisite 30Β causes a decrease of T max in the ABS/PC 70/30 w/w blend, which becomes more clear as the clay content increases, whereas for 50/50 and 30/70 w/w blends, the clay load does not essentially affect T max values.
From Fig. 4, it can be observed that the addition of clay in 50/50 w/w ABS/PC blends causes significant changes to the thermal degradation mechanism that takes place in two stages, whereas the degradation of non-reinforced blends proceeds in one single stage. In fact, a peak broadening toward the high temperature area was obvious in the case of nanocomposites.
With respect to the ABS/PC 30/70 w/w blend, the incorporation of Cloisite 30B into the polymer matrix further splits the degradation peak into two and enhances the thermal resistance at the second step (Fig. 4). The improvement of thermal characteristics of ABS/PC blends at 50/50 and 30/70 w/w is attributed to the better intercalation of montmorillonite silicate layers taking place in these polymer matrices, in comparison with 70/30 w/w ABS/PC blends, as confirmed with XRD analysis. Cloisite 30B seems to inhibit the thermal degradation of the PC phase, which might be due to the formation of a protective layer of nanoclay at the ABS/PC interface or to the creation of new paths for the degradation reaction.
Wang et al. [24] prepared and studied PC/ABS blends at proportions 3/2 and 4/1 and their nanocomposites with 5 mass%. The above authors attributed the two-stage decomposition of nanocomposites to the nanodispersed lamellae of clay into the polymer matrix, which may change the decomposition process of polymer by acting as an excellent thermal insulator and mass transport barrier. Feyz et al. [27] observed that the decomposition of PC/ABS 65/35 w/w is a single-step process, whereas that of PC/ABS with nanoclay is a two-step process, with the first step in the range of 400–500 °C and the second step in that of 500–600 °C. They explained this behavior by the interaction between nanoclay and polymer matrix, which can lead to a change of the thermal stability of ABS resin and turn the degradation mechanism into a two-step process.
The incorporation of organoclay reinforcement seems to increase the char residue (Table 4), offering some “protection” to the polymer matrix during thermal degradation. However, the increase in char residue is not sensitive against the amount of the added reinforcement.
Gross calorific value
Effect of ABS and PC proportion
From Table 5, it can be seen that ABS has a higher gross calorific value (GCV) in comparison with PC. It was also shown that the gross calorific values for pure ABS and PC (39.9 and 32.2 kJ g−1, respectively) obtained experimentally in this study are similar to those of literature and the technical data from Bayer (GCVABS = 40 kJ g−1 and GCVPC = 31 kJ g−1) [28]. Furthermore, according to Table 5, the extruded ABS and PC (42.4 and 37.5 kJ g−1, respectively) present higher values of GCV than those corresponding to virgin polymers.
Regarding the ABS/PC blends, as expected, the GCV is reduced with increasing PC content. The ABS/PC 70/30 w/w blend presents a positive deviation from the rule of mixtures, whereas the opposite effect was observed for the ABS/PC 50/50 and 30/70 w/w blends.
The theoretical gross calorific value for the examined polymers was calculated using the methods developed on “Combustion thermochemistry” section, and the obtained results are presented in Table 6. The theoretical GCV values corresponding to ABS and PC by the different examined models are similar and close to the experimental data obtained for the virgin materials.
Using an oxygen bomb calorimeter, Walters et al. [15] found that the gross heat of combustion of ABS and PC is 39.84 and 31.3 kJ g−1, respectively. They compared the experimental results with thermochemical calculations of the gross heat of combustion, from group additivity of the heats of formation of products and reactants, and found excellent agreement (group contribution gross for ABS and PC is 39.43 and 31.2 kJ g−1, respectively). Othman et al. [29] calculated the higher heating value of waste ABS, PC, and their blends using the Dulong equation and found 35.16, 26.71, and 29.72 kJ g−1, respectively. From the analysis, they concluded that electronic plastic waste is a potential fuel to recover energy, due to the high hydrogen and carbon content.
Effect of clay loading
The incorporation of OMMT into ABS and 70/30 w/w blend ABS/PC increases slightly their gross calorific value for filler concentrations up to 2 phr, followed by a decrease at 3 phr clay loading. Blends with higher PC content (50/50 and 30/70 w/w ABS/PC) present a slight decrease in GCV at almost all the examined clay concentrations. The values of GCV follow an inverse trend than that observed for the residue after thermogravimetric analysis of the examined nanocomposites. Therefore, it seems that some relationship exists between GCV and the residue after thermogravimetric analysis, since the latter does not contribute to the released calorific value during combustion of the specimens, due to the protective mechanism developed by the organoclay platelets, as mentioned above. Deviation from the above behavior was noticed for ABS nanocomposites with organoclay content up to 2 phr and can be attributed to the relatively low residue after TG experiment, which does not have any obvious effect on GCV.
Correlation between thermal degradation mechanism and gross calorific value in ABS/PC blend nanocomposites
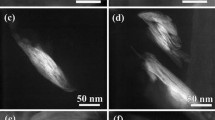
Based on the above experimental results and on the literature survey, the following scheme is proposed regarding the thermal degradation mechanism of 50/50 ABS/PC nanocomposite. According to SEM analysis, this blend consists of a continuous ABS phase with a dispersed PC phase (Fig. 5). The size reduction of the average microdomain of PC, attributed to the addition of organoclay, can be observed by comparing the image corresponding to the reinforced system (Fig. 5b) with that of the unreinforced material (Fig. 5a). This difference might suggest that the above additive improves the compatibility of the investigated ABS/PC blends. Clay tactoids are arranged mainly on the ABS/PC interphase, which promotes miscibility of the two components, whereas a significant amount is located in the ABS phase due to the affinity between the polar nitrile groups of ABS with the organic modification of montmorillonite. As shown from XRD spectra, the nanodispersed silicate layers interact with the polymer matrix. The formation of a physical crosslinked structure might be possible, composed of clay nanoparticles and polymeric chains. This results to the limitation of chain mobility and to their protection by clay platelets during the thermal degradation process. Moreover, the dispersion of montmorillonite layers leads to the formation of a tortuous path, which inhibits the passage of volatile degradation products during the initiation of thermal degradation (labyrinth effect) (Fig. 6a).
As the thermal degradation proceeds (Fig. 6b), the ABS phase which is located at the surface decomposes more rapidly, since ABS presents lower thermal stability than PC, thus leaving the layered silicates to remain on the surface. In addition, the layered silicates, pushed by numerous rising bubbles of degradation products, are transported to the surface. Thus, a carbonaceous silicate char builds up on the surface during burning, which insulates the underlying material creating a protective barrier to heat and mass transfer (barrier effect).
The heat barrier effect could also provide superheated conditions inside the polymer melt, leading to extensive random chain scission and evolution of numerous chemical species, which in turn, are trapped between the clay layers and are likely to undergo secondary reactions [7]. As a result, some degradation pathways could be promoted leading to enhanced charring (Fig. 6c). In the case where the promoted pathway leads to higher molecular weight material, the polymer degrades more slowly than in the absence of clay. From TG results, it was observed that the incorporation of OMMT in 50/50 ABS/PP blends causes significant changes to the thermal degradation mechanism, which turns into a two-stage process, whereas the degradation of the respective unreinforced blend proceeds in one stage. In fact, a peak broadening toward the high temperatures area is obvious in the case of nanocomposites. In addition, the clay may initiate radical recombination reactions, exerting thus a stabilization effect in the ABS/PC/layered silicate nanocomposite.
In conclusion, the improvement of thermal stability of ABS/PC nanocomposites is attributed to the condensed-phase thermal degradation retardant mechanism [6, 30, 31]. A measure of this improvement might be the increase of residue yield and the decrease of gross calorific value (Fig. 6d).
Conclusions
The incorporation of PC improves the thermal resistance of ABS/PC blends, with respect to pure ABS. The addition of OMMT alters the degradation mechanism and modifies the thermal stability of blends with higher PC content, i.e., 50/50 and 30/70 w/w ABS/PC blends, where a shift of the PC phase degradation temperature to higher values becomes obvious, in comparison with the respective unreinforced blends. This behavior was attributed to the better intercalation of montmorillonite silicate layers taking place in these polymer matrices in comparison with 70/30 w/w ABS/PC blends, as confirmed by XRD analysis
In most of the examined nanocomposites, an inverse trend was observed between the calculated gross calorific value and the residue remaining after thermogravimetric analysis in inert atmosphere. Therefore, it seems that some relationship exists between GCV and the residue, since the latter does not contribute to the released calorific value during combustion of the specimens, due to a possible inhibition effect caused by the organoclay platelets. Based on the above results, the thermal degradation behavior of ABS/PC nanocomposites was interpreted by the heat barrier effect due to the presence of a carbonaceous silicate char, which insulates the underlying material creating a protective barrier to heat and mass transfer.
References
Levchik SV, Weil ED. A review of recent progress in phosphorus-based flame retardants. J Fire Sci. 2006;5:345–64.
Perrer B, Pawlowski KH, Schartel B. Fire retardancy mechanisms of arylphosphates in polycarbonate (PC) and PC/acrylonitrile–butadiene–styrene. J Therm Anal Calorim. 2009;97:949–58.
Feyz E, Jahani Y, Esfandeh M. Effect of a nanoclay/triphenyl phosphate hybrid System on the fire retardancy of polycarbonate/acrylonitrile–butadiene–styrene blend. J Appl Polym Sci. 2011;120:3435–42.
Qin H, Zhang S, Zhao C, Hu G, Yang M. Flame retardant mechanism of polymer/clay nanocomposites based on polypropylene. Polymer. 2005;46:8386–95.
Gilman JW. Flammability and thermal stability studies of polymer layered-silicate (clay) nanocomposites. Appl Clay Sci. 1999;15:31–49.
Gilman JW, Jackson CL, Morgan AB, Harris R Jr, Manias E, Giannelis EP, Wuthenow M, Hilton D, Philips SH. Flammability properties of polymer-layered silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem Mater. 2000;12:1866–73.
Leszczyńska A, Njuguna J, Pielichowski K, Banerjee JR. Polymer/montmorillonite nanocomposites with improved thermal properties Part I. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim Acta. 2007;453:75–96.
Zanetti M, Kashiwagi T, Falqui L, Camino G. Cone calorimeter combustion and gasification studies of polymer layered silicate nanocomposites. Chem Mater. 2002;14:881–7.
Lomakin SM, Dubnikova IL, Shchegolikhin AN, Zaikov GE, Kozlowski R, Kim G-M, Michler GH. Thermal degradation and combustion behavior of the polyethylene/clay nanocomposite prepared by melt intercalation. J Thermal Anal Calorim. 2008;94:719–26.
Ma H, Tong L, Xu Z, Fang Z. Clay network in ABS-graft-MAH nanocomposites: rheology and flammability. Polym Degrad Stab. 2007;92:1439–45.
Barbosa R, Alves TS, Araújo EM, Mélo TJA, Camino G, Fina A, Ito EN. Flammability and morphology of HDPE/clay nanocomposites. J Therm Anal Calorim. 2014;115:627–34.
Kannan M, Bhagawan SS, Thomas S, Joseph K. Thermogravimetric analysis and differential scanning calorimetric studies on nanoclay-filled TPU/PP blends. J Thermal Anal Calorim. 2013;112:1231–44.
Zhu J, Uhl FM, Morgan AB, Wilkie CA. Studies on the mechanism by which the formation of nanocomposites enhances thermal stability. Chem Mater. 2001;13:4649–54.
Galvan D, Carneiro F, Mazzucco M, Bartoli JR, Akira d’Avila M, Morales AR, Fernandes EG. Effect of organoclay mixture on the rheological properties of ABS-clay nanocomposites. Macromol Symp J. 2012;319:167–72.
Walters RN, Hackett SM, Lyon RE. Heats of combustion of high temperature polymers. Fire Mater. 2000;24:245–52.
Van Krevelen DW, TeNijenhuis K. Properties of polymers, 4th revised ed. New York: Elsevier; 2009.
Walters RN. Molar group contributions to the heat of combustion. Fire Mater. 2002;26:131–45.
Meraz L, Dominguez A, Kornhauser I, Rojas F. A thermochemical concept-based equation to estimate waste combustion enthalpy from elemental composition. Fuel. 2003;82:1499–507.
Lee KM, Han CD. Effect of hydrogen bonding on the rheology of polycarbonate/organoclay nanocomposites. Polymer. 2009;44:4573–88.
Ambre A, Jagtap R, Dewagan B. ABS nanocomposites containing modified clay. J Reinf Plast Compos. 2009;28:343–52.
Lim SK, Hong EP, Song YH, Park BJ, Choi HJ, Chin IJ. Preparation and interaction characteristics of exfoliated ABS/organoclay nanocomposite. Polym Eng Sci. 2010;50:506–12.
Hong JH, Sung YT, Song KH, Kim WN, Kang BI, Kim SL, Lee CH. Morphology and dynamic mechanical properties of poly(acrylonitrile–butadiene–styrene)/polycarbonate/clay nanocomposites prepared by melt mixing. Compos Interfaces. 2007;14:519–32.
Zong R, Hu Y, Liu N, Wang S, Liao G. Evaluation of the thermal degradation of PC/ABS/montmorillonite nanocomposites. Polym Adv Technol. 2005;16:725–31.
Wang S, Hu Y, Wang Z, Yong T, Chen Z, Fan W. Synthesis and characterization of polycarbonate/ABS/montmorillonite nanocomposites. Polym Degrad Stab. 2003;80:157–61.
Nigam I, Nigam D, Marthur GN. Effect of rubber content of ABS on properties of PC/ABS blends. I. Rheological, mechanical, and thermal properties. Polym-Plast Technol Eng. 2005;44:815–32.
Balart R, Sánchez L, López J, Jiménez A. Kinetic analysis of thermal degradation of recycled polycarbonate/acrylonitrile-butadiene-styrene mixtures from waste electric and electronic equipment. Polym Degrad Stab. 2006;91:527–34.
Feyz E, Jahani Y, Esfandeh M. Comparison of the effect of an organoclay, triphenylphosphate, and a mixture of both on the degradation and combustion behaviour of PC/ABS blends. Macromol Symp. 2010;298:130–7.
Bayer, Macrolon and Vivak Environmental Aspects. Technical information, June 2004.
Othman N, Basri NEA, Yunus MNM, Sidek LM. Determination of physical and chemical characteristics of electronic plastic waste (Ep-waste) resin using proximate and ultimate analysis method. ICCBT. 2008;D16:169–80.
Ozkaraca AC, Kaynak C. Contribution of nanoclays to the performance of traditional flame retardants in ABS. Polym Compos. 2012;33:420–9.
Zong R, Hu Y, Wang S, Song L. Thermogravimetric evaluation of PC/ABS/montmorillonite nanocomposite. Polym Degrad Stab. 2004;83:423–8.
Acknowledgements
We would like to acknowledge the Bodossaki Foundation for its financial support. Special thanks go to Prof. D. Karonis and Dr A. Deligiannidis, Lab. of Lubricants and Fuels, School of Chemical Eng. NTUA, for carrying out the Gross Calorific value measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Triantou, M.I., Chatzigiannakis, E.M. & Tarantili, P.A. Evaluation of thermal degradation mechanisms and their effect on the gross calorific value of ABS/PC/organoclay nanocomposites. J Therm Anal Calorim 119, 337–347 (2015). https://doi.org/10.1007/s10973-014-4152-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4152-1