Abstract
Bio-waste material-assisted nanomaterials synthesis is a newer aspect of modern nanotechnology. It is preferred over orthodox approaches for its merits, including affordability, safety, eco-benevolentness, and biocompatibility. In this report, the green fabrication of heterogeneous Cu/CuO/Cu2O NPs using groundnut shell extract and assessed for their diverse biomedical properties. Diverse spectroscopic and microscopic characterization techniques such as X-ray diffraction (XRD), Fourier-transformed infrared spectroscopy (FTIR), scanning electron microscope (SEM), energy-dispersive X-ray spectroscopy (EDX) and transmission electron microscope (TEM) were performed to confirm the synthesis of groundnut shells extract mediated Cu/CuO/Cu2O NPs. XRD data demonstrated a high degree of crystallinity of the Cu/CuO/Cu2O NPs and a median diameter of about 34 nm. HRTEM and SEM images disclosed the quasi-spherical morphology of NPs. FTIR study ascertained the involvement of diverse functional groups that reduced and stabilized the Cu/CuO/Cu2O NPs. Moreover, the anticancer efficacy of synthesized Cu/CuO/Cu2O NPs was evaluated against breast cancer cell lines (MCF-7) through an MTT assay and displayed activity at the IC50 of 42.66 μg/mL. Antioxidant properties of Cu/CuO/Cu2O NPs were surveyed through the ABTS and DPPH assays. Furthermore, the agarose gel electrophoresis technique has scrutinized the DNA cleavage abilities of the as-prepared Cu/CuO/Cu2O NPs. Hence, the present green approach represents a facile, greener, safe and cost-effective route for synthesizing Cu/CuO/Cu2O NPs and opening new horizons for the biomedical sector.
Graphical Abstract

Bio-waste material-aided nanomaterials fabrication is a novel aspect of advanced nanotechnology. It is preferred over orthodox approaches for its merits, including simplicity, affordability, reliability, eco-merciful, and biocompatibility. In this context, the facile green synthesis of heterogeneous Cu/CuO/Cu2O NPs via groundnut shell extracts and explored for their various biomedical applications. Diverse spectroscopic and microscopic characterization techniques such as XRD, FTIR, SEM, EDX, and TEM were employed to confirm the biogenic synthesis of groundnut shells extract-mediated Cu/CuO/Cu2O NPs. Moreover, the antioxidant, anticancer, and DNA damage potential of the as-synthesized NPs was carefully studied. Therefore, the current green approach represents a facile, greener, biocompatible, safe, and cost-effective protocol for synthesizing Cu/CuO/Cu2O NPs and opening a new arena in the domain of nanomedicine.
Highlights
-
First-time report on groundnut shell extracts-mediated synthesis of heterogeneous Cu/CuO/Cu2O NPs through a cost-effective and eco-friendly approach.
-
Optical, structural, and topological properties of Cu/CuO/Cu2O NPs were studied using XRD, FTIR, SEM, EDX and HRTEM techniques.
-
As-prepared Cu/CuO/Cu2O NPs exhibited effective cytotoxic activity against breast cancer cell lines (MCF-7) using MTT assay.
-
Moreover, antioxidant and DNA cleavage studies of Cu/CuO/Cu2O NPs investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Presently, modern nanotechnology is considered a most emerging research domain for synthesizing, visualizing, and modifying materials at the nanoscale [1,2,3]. It leads to structural modifications and implementation, and its swift synthesis has grabbed substantial interest worldwide [4, 5]. The confluence of biological platforms and nanomaterials enables these technological advances to interconnect, offering creation to the nascent domain of nano-biotechnology [6]. Modern nano-biotechnology acquired a plethora of scientific curiosity due to the use of nanoscale materials/devices in diagnosing biological processes [7, 8]. The primary feature possessions of NPs are their tiny size and large surface area, which allow massive concentration to acquire their chemical, magnetic, optoelectronic, and physical characteristics [9, 10]. The essential features, such as biological, optical absorption, catalytic, electrical, and thermal conductivity, etc., are endowed on a vast scale by the regulated experiments of shape and size distribution of nanoparticles (NPs) [11,12,13]. Moreover, metal-based NPs were known for their usefulness in textiles, sensors, catalysis, energy harvesting, food packaging, fertilizers, wastewater treatment, drug delivery, and medical diagnosis [14,15,16,17,18,19].
Coinage metal copper (Cu) belongs to the 3d transition series and has intriguing physicochemical features. Recently, Cu-based materials have been extensively studied for a plethora of purposes in material science due to the different accessible oxidation states (Cu0, Cu+1, Cu+2, and Cu+3) and the high conductivity of Cu metal [19, 20]. Also, Cu is an inexpensive, high abundance, and non-toxic material that could be implemented in environmental remediation and pharmaceutical applications [21,22,23,24,25]. Because of their intriguing features, Cu-based nanomaterials have been explored for diverse usefulness in advanced nanotechnology, including the biomedical sector, sensors, catalysis, solar cells, environmental remediation [19, 20, 22, 26, 27], biosensors [28], gene therapy [29], and antibacterial applications [30]. Amidst Cu-based nanomaterial, Cu/CuO/Cu2O NPs have noteworthy interest from material scientists due to their splendid applications, such as sensing [31,32,33], fuels [34], photocatalysis [35], lithium-ion batteries [36], and oxygen evolution reaction [37]. Furthermore, they can be used biomedical sector [38] and wastewater treatment [39]. To date, Cu/CuO/Cu2O material has been fabricated through diverse approaches listed in the literature, such as hydrothermal [34, 35], exploding wire technique [40], aerosol furnace reactor [32], electrochemical [37], annealing [41], sol-gel [42], and green method [31], etc. The approaches listed above are reliable and facile, but they frequently need deleterious organic solvents, non-biodegradable stabilizers, noxious chemicals, expensive equipment, and time-consuming operation [20]. As a corollary, there is an enormous need for a greener approach to synthesizing NPs [19]. Therefore, a plethora of greener approaches, including biopolymers, plants, fibres, bacteria, enzymes, fungi, bio-waste material, etc., are being sought to produce NPs through biological sources [8]. Additionally, biologically produced NPs are more stable, robust, and biocompatible when likened to chemically prepared NPs. Using biological sources in association with the expedited protocol was noticed as a more sustainable, safer, inexpensive, and ecologically acceptable approach from the standpoint of green synthesis [8, 9, 19].
Groundnut shell is one of the most common, cheapest, natural, and agro bio-waste materials pervasive in Asian and African nations. Endeavours are being explored for a divergent applications, including dietary fiber for humans [43], animal feed products for cattle [44], biofuel production [45], pulp production [46], particle board component [47], and utilized as fuel [48] in diverse industries, including textile, food processing, pharmaceutical, etc. As a result of their intrinsic features like non-toxicity, eco-benevolentness, longer burning period, and smokeless and lower ash content, these items have gotten in great demand in the industrial sector [49]. Additionally, groundnut shells have valuable pharmacological and nutritional properties due to the active biomolecules (Fig. 1); this is why they are employed to make antibacterial and anti-aging agents [48]. Therefore, our goal in the current study is to develop a large-scale synthesis of NPs for potential use in the biomedical sector. This approach has no negative effect on the environment and effectively manages waste as the waste is being employed as an initial source. In that context, the current study reports for the first time on the bio-inspired fabrication of Cu/CuO/Cu2O NPs through a green chemistry approach using groundnut shell extract as a natural reducing/stabilizing agent. The characterizations of the produced NPs were revealed through XRD, FTIR, SEM, EDX, and HRTEM techniques for studying their physicochemical properties critically. In addition, the antioxidant, anticancer, and DNA damage potential of the synthesized NPs was also carefully explored.
2 Experimental
2.1 Material
The groundnut shells were sourced from the nearby market of Nashik, India. Copper acetate monohydrate (Cu(CO2CH3)2.H2O, 99%) was procured from SRL Chem, India, and utilized in the experiment. All additional chemicals associated with this work were of the AR grade. The glassware was properly rinsed with chromic acid and acetone prior to use. It was then carefully rinsed three times with double-distilled water (ddH2O) and dried in a hot air oven.
2.2 Preparation of groundnut shell extract
The obtained groundnut shells were cleaned with ddH2O to remove the dust and dried in a hot oven at 80 °C for 24 h to altogether remove the humidity. After that, the wizened groundnut shells were finely ground using a domestic blender. Exactly 5.0 g of this powder was taken in a 250 mL beaker containing 100 mL ddH2O and then boiled for 20 min at 85–90 °C. After cooling, the solution was filtered at room temperature (RT) through Whatman No. 40 filter paper. The resulting filtrate (groundnut shell extract) was collected in a beaker, stored at 4 °C, and used to prepare Cu/CuO/Cu2O NPs.
2.3 Bio-inspired production of Cu/CuO/Cu2O NPs
The bio-inspired fabrication of Cu/CuO/Cu2O NPs was accomplished through an entirely green chemistry protocol. To prepare Cu/CuO/Cu2O NPs, 1.9 g of copper acetate was mixed with 100 mL groundnut shell extract. Further, the reaction mixture was stirred continuously on a magnetic stirrer at RT for 30 min. The color change in the solution confirmed the reduction process of Cu salt. The blackish precipitate of Cu/CuO/Cu2O NPs was collected through centrifugation at 3000 rpm for 10 min. The collected material was washed through isopropanol followed by ddH2O and dried in a hot air oven for 5 h at 100 °C. The NPs were further calcined in a muffle furnace at 400 °C for 2 h. The blackish NPs powder of Cu/CuO/Cu2O NPs was finally collected and stored in an airtight Eppendorf at RT for further study. A schematic illustration of Cu/CuO/Cu2O NPs synthesis is portrayed in Fig. 2.
Graphical presentation for the bio-inspired production of Cu/CuO/Cu2O NPs from groundnut shells extract (www.biorender.com)
2.4 Characterization tools
Diverse characterization tools were applied to study the textural properties of Cu/CuO/Cu2O NPs. The crystallinity, morphology, size, and functional group analysis of Cu/CuO/Cu2O NPs were investigated through X-ray diffraction (XRD, Panalytical X’PERT PRO), Fourier transform infrared spectroscopy (FTIR, 4600 typeA), scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDX) (VEGA3 TESCAN) and high-resolution transmission electron microscopy (HRTEM, JEM-2100).
2.5 Anticancer activity
Using the MTT protocol, the anticancer efficacy of Cu/CuO/Cu2O NPs against breast cancer cells line (MCF-7) was assessed [50, 51]. The entire protocol for the anticancer study was described in our earlier work [50]. Herein, a 96-well plate cultured in DMEM/RPMI containing 10% inactivated FBS, penicillin (100 IU/mL), and streptomycin (100 g/mL) at 37 °C with 5% CO2. MCF-7 cells (50,000/well) were seeded in a 96-well plate and allowed to grow overnight at 37 °C. The various concentrations of Cu/CuO/Cu2O NPs were treated with the cells for 24 h. Afterward, 100 µL of the MTT reagent was added to each well and incubated for 4 h at 37 °C. After 15 min of shaking, 100 µL of dimethyl sulfoxide was poured into each well to dissolve the formazan crystals. The absorbance was measured utilizing an ELISA microplate reader at 570 nm. The sample without adding the Cu/CuO/Cu2O NPs consider a positive control. The concentration of Cu/CuO/Cu2O NPs needed to prevent 50% of viability (IC50) was obtained microscopically, and the cell viability percentage was computed employing the following equation.
2.6 Antioxidant efficacy
The scavenging effect of greenly synthesized Cu/CuO/Cu2O NPs was determined by two diverse assays: the ABTS and DPPH radical assay. The detailed protocol for the antioxidant capacity of both assays was described in our earlier work [50]. For this study, diverse concentrations (20–100 μg/mL) of Cu/CuO/Cu2O NPs were used. Ascorbic acid was applied as a positive control to calibrate the resultant scavenging effect. The percentage of inhibition of ABTS and DPPH assays was computed using the formula as follows:
2.7 DNA damage study
The agarose gel electrophoresis technique was accomplished to ascertain the DNA damage efficacy of the biogenically produced Cu/CuO/Cu2O NPs. The complete protocol for the DNA damage study was explained carefully in our earlier work [50].
3 Results and discussion
3.1 XRD analysis
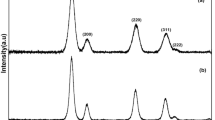
In order to ascertain the existence of different phases of Cu in the biosynthesized NPs using aqueous extract of groundnut shell bio-waste, the XRD analysis of Cu/CuO/Cu2O NPs was accomplished, and the result is illustrated in Fig. 3. The assigned diffraction peaks, in Fig. 3, at 2θ values of 32.5°, 35.5°, 38.7°, 48.8°, 53.5°, and 58.3° corresponds to (110), (111), (200), \(\left( {\overline 2 02} \right)\), (020), and (202) diffraction planes and represent the existence of end-centred monoclinic crystalline phase of CuO NPs [52]. This data was asserted by the International Centre for Diffraction Data (ICDD) file no. 80-1916. The peaks marked by * in Fig. 3 at 2θ values of 36.4°, 42.3°, and 61.5°, belong to (111), (200), and (220) diffraction planes of Cu2O NPs (ICDD file no. 05-0667) [53]. The peaks marked by # in Fig. 3 centred at 2θ values of 43.3 and 50.5 represents (111) and (200) diffraction planes of Cu NPs (ICDD card no. 04-836) [53]. These results indicate the existence of three different phases of Cu, i.e., CuO, Cu2O, and Cu NPs, in the as-synthesized sample. Notably, the higher intensity of CuO diffraction peaks indicates that CuO NPs is the dominant phase in this sample. The median crystallite size of the Cu/CuO/Cu2O NPs was computed using Scherrer’s equation [54] and was found to be 33.8 nm. Thus, the successful formation of Cu/CuO/Cu2O NPs through a bio-inspired route was confirmed by XRD analysis.
3.2 FTIR analysis
In this study, the Cu/CuO/Cu2O NPs were biosynthesized using the extract of groundnut shells. This extract of bio-waste material acts as the capping agent and prevents the agglomeration of as-prepared Cu/CuO/Cu2O NPs. In order to uncover the functionalization of groundnut shell extract onto the surface of CuO Cu/CuO/Cu2O NPs, FTIR spectra of both the groundnut shell (bio-waste) and Cu/CuO/Cu2O NPs were studied, and the results are given in Fig. 4. It may be observed from Fig. 4 that all the major FTIR peaks of the groundnut shell are present in the as-prepared Cu/CuO/Cu2O NPs, indicating the successful capping function of the former. The only difference that may be observed from the FTIR spectra is the intensity of the peaks. This difference indicates that the cellulose, lignin, pectin, and hemicellulose, major active biomolecules of a bio-waste were successfully adsorbed onto the surface of Cu/CuO/Cu2O NPs through either electrostatic attraction or complexation mechanism during synthesis [55].
The peak observed at 2927.9 cm−1 corresponds to the C-H stretching vibration vibrations of lignocellulosic molecules, while the peak at 2288.5 cm−1 belongs to C ≡ C stretching frequency [55, 56]. The peaks centred at 1618.2 and 1508 cm−1 are due to the C = O group of hemicelluloses and lignin present in the groundnut shell [55, 56]. The peak at 1386.3 cm−1 may be attributed to the bending vibration of C=C, while the peak at 1032.1 cm−1 may be ascribed to the C-O bending vibration [55]. Further, a peak at 517.8 cm−1 may be observed at the FTIR spectrum, representing Cu-O stretching along the [101] direction [57].
3.3 SEM and EDX analysis
The topology of the NPs determines their surface properties. SEM characterization was conducted to analyze the surface property of the as-prepared Cu/CuO/Cu2O NPs. Figure 5a represents the SEM image of Cu/CuO/Cu2O NPs. It may be observed from the figure that most of the particles have block-like structures. At the same time, smaller particles are also present, which appear to have broken down from a large chunk. This implies that the particles of the as-synthesized Cu/CuO/Cu2O NPs have agglomerated under biosynthesis to form block-like structures. Figure 5b represents the EDX spectra of the groundnut shell functionalized Cu/CuO/Cu2O NPs with the weight and atomic percentages details in a tabular format. From this spectrum, the highest weight percent is for Cu and O elements indicating the successful formation of Cu/CuO/Cu2O NPs. It may also be noted that the atomic percentage of C is comparatively high than the other elements except Cu. Using the carbon grid may justify the higher percentage of C in the sample during the EDX analysis. Further, this carbon contributes to the bio-waste extract, as seen from the FTIR spectra. The remaining elements observed from the EDX spectra may be the constituents of the bio-waste extract.
3.4 HRTEM analysis
The microstructural analysis of the biosynthesized bio-waste functionalized Cu/CuO/Cu2O NPs was performed using the HRTEM technique. Figure 6a represents the TEM image of Cu/CuO/Cu2O NPs. From this image, it is difficult to decipher the exact microstructure of the NPs. However, agglomeration in the structure of Cu/CuO/Cu2O NPs may be noted. Figure 6b represents the HRTEM image of Cu/CuO/Cu2O NPs at a magnification of 10 nm. In this figure, it may be observed that the particles of Cu/CuO/Cu2O possess quasi-spherical microstructure. The average particle size of the as-synthesized Cu/CuO/Cu2O NPs was calculated to be 5.3 nm. It is evident that there is an inconsistency in the size of the particles as calculated through SEM and TEM techniques since the former presents the morphology of the sample while the latter gives the microstructural analysis of the sample. Figure 6c represents the HRTEM image of Cu/CuO/Cu2O NPs at a magnification of 2 nm. At this magnification scale, the lattice fringes of Cu/CuO/Cu2O NPs are prominent.
From this image, d-spacing (or inter-planar spacing) between two lattice fringes was calculated to be 0.210 nm which is in agreement with the reported literature value of the (111) plane of monoclinic CuO [58]. Figure 6d represents the selected area electron diffraction (SAED) pattern of the as-prepared Cu/CuO/Cu2O NPs. From the SAED pattern, the appearance of bright spots and not fused rings may be noted, indicating this sample’s polycrystalline nature. Therefore, the results of the SAED pattern matches well with that of the XRD data considering the highly crystalline nature of the sample.
3.5 Anticancer study
Breast cancer (MCF-7) cells were used to explore the anticancer potential of biosynthesized Cu/CuO/Cu2O NPs at varying doses (10–320 µg/mL). The groundnut shells extract-mediated synthesized Cu/CuO/Cu2O NPs had potential anticancer performance against the MCF-7 cancer cell line, relying on the concentration of NPs. The cell viability (Fig. 7) exponentially decreases if an increasing the concentration of Cu/CuO/Cu2O NPs; the lower concentration of 10 μg/mL revealed 82.49% cells of viability for MCF-7. Synthesized Cu/CuO/Cu2O NPs displayed (Fig. 8) half maximal inhibition concentration (IC50) against MCF-7 cells at 42.66 μg/mL, while Doxorubicin revealed 27.19 µg/mL. The results of our study were well accompanied by different study reports on the anticancer performance of the greenly produced Cu-based NPs employing the extracts of Lonicera caprifolium [59], Wrightia tinctoria [60], Prunus nepalensis [61], Sambucus nigra [62], and Prosopis cineraria [63]. However, biogenically fabricated other types of NPs using diverse plant extracts have been studied to investigate anticancer performance [64, 65].
Also, NPs are promising for cancer research in many biomedical realms, particularly as drug-delivery vehicles in cancer treatment. The training of the NPs’ functionalities, including their chemical and biological potential, is considerably affected by their size. A fascinating part of nanobiotechnology is understanding how NPs work as an anticancerous agent. The channels of the mechanism of cytotoxic actions involve (a) direct physical interaction of highly sharp edges of NPs with cell wall membrane [66], (b) reactive oxygen species (ROS) generation [67, 68], (c) trapping the cells within the aggregated NPs [69], (d) oxidative stress [70], (e) DNA damaging and chromosomal aberration [71], (f) ion release [72], (g) contribution in generation/explosion of nanobubbles [73], and h) affecting the hormonal secretion [74]. These mechanistic modes are crucial for improving the pharmacological delivery system in cancer therapy.
3.6 Antioxidant efficacy
Antioxidant effectiveness is an essential and key exploration of nanomaterials [75]. Antioxidants perform a significant aspect in all living things. Free radicals are created in biological processes due to reactions between biomolecules and molecular oxygen [61]. In this study, the percentage inhibition of biogenically fabricated Cu/CuO/Cu2O NPs at various concentrations demonstrated good antioxidant potential (Fig. 9). According to the results, the IC50 values for Cu/CuO/Cu2O NPs’ abilities to scavenge ABTS and DPPH are 78.17 and 95.51 µg/mL, respectively. Cu/CuO/Cu2O NPs had the highest scavenging inhibition for the ABTS and DPPH assays of 42.38 and 8.56% at 100 µg/mL, respectively. The scavenging performance of the Cu/CuO/Cu2O NPs increases exponentially with increased concentration. The high scavenging performance of Cu/CuO/Cu2O NPs is primarily a result of their redox characteristics [23, 75]. According to the results of methods for scavenging free radicals, Cu/CuO/Cu2O NPs had much more scavenging activity for the ABTS assay than the DPPH assay.
3.7 DNA damage capacity
The potential of Cu/CuO/Cu2O NPs to cleave DNA was evaluated through agarose gel electrophoresis. It is an effective method for identifying DNA damage. DNA has broken an area on the genome at a specified sequence during agarose electrophoreses for DNA typing, and the results are compared to a control model. As a result, it can also be used to assess DNA damage. The transformation of pBR 322 DNA’s supercoiled circular conformation (Form I) to nicked circular conformation (Form II) and linear conformation (Form III) serves as a DNA cleavage check. Figure 10 shows the overall result. Cu/CuO/Cu2O NPs demonstrated nuclease efficacy at all tested concentrations. Control experiments using pBR 322 plasmid DNA failed to show any DNA cleavage activity (Lane 1). At 1 μL, the supercoiled plasmid DNA was transformed into a circular shape. However, greater concentrations (3 μL) showed that Form I was transformed into Form III. We ascertained from the results that Cu/CuO/Cu2O NPs served as an efficient chemical nuclease for cleaving double-strand DNA. This outcome demonstrated that, after passing toxicologic test systems, Cu/CuO/Cu2O NPs could be considered an optional cancer treatment as a DNA target agent. However, recently few biogenically produced NPs have been explored for DNA damage ability [76, 77].
4 Conclusion
The bio-inspired synthetic approach was applied to synthesize Cu/CuO/Cu2O NPs, and the groundnut shell was assigned as a reducing/stabilizing candidate in our study. It was asserted that the groundnut shell extract could be effectively implemented to reduce Cu metal at room temperature. The textural properties of the groundnut shell-mediated Cu/CuO/Cu2O NPs were explored through XRD, FTIR, SEM, EDX, and HRTEM techniques. It was noticed that synthesized Cu/CuO/Cu2O NPs displayed excellent anticancer effects against MCF-7 cancer cell lines after 24 h of treatment with increasing concentrations and showed IC50 value at 42.66 μg/mL. However, Cu/CuO/Cu2O NPs have shown efficacious scavenging performance and DNA cleavage ability. This result revealed that the biogenically synthesized NPs are highly stable and cheaper with significant biological applications, which may be used effectively in the biomedical sector for safe drug delivery.
References
Hansen SF et al. (2022) Nanotechnology meets circular economy. Nat Nanotechnol 17(7):682–685
Pushparaj K et al. (2022) Nano-from nature to nurture: A comprehensive review on facets, trends, perspectives and sustainability of nanotechnology in the food sector. Energy 240:122732
Woolley JL, MacGregor N (2022) Science, technology, and innovation policy timing and nanotechnology entrepreneurship and innovation. Plos one 17(3):e0264856
Ghotekar S (2019) A review on plant extract mediated biogenic synthesis of CdO nanoparticles and their recent applications. Asian J Green Chem 3(2):187–200
Yang RX et al. (2022) Big data in a nano world: A review on computational, data-driven design of nanomaterials structures, properties, and synthesis. ACS Nano 16(12):19873–19891
Malik S et al. (2022) A comprehensive review on nanobiotechnology for bioremediation of heavy metals from wastewater. J Basic Microbiol 62(3–4):361–375
Jiang T et al. (2022) Nanobiotechnology: Applications in Chronic Wound Healing. Int J Nanomed 17:3125–3145
Pansambal S et al. (2022) Bioengineered cerium oxide (CeO2) nanoparticles and their diverse applications: A review. Appl Nanosci 1–26
Ghotekar S et al. (2023) Recent Advances in Synthesis of CeVO4 Nanoparticles and Their Potential Scaffold for Photocatalytic Applications. Topics Catalysis 66:89–103
Baig N, Kammakakam I, Falath W (2021) Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater Adv 2(6):1821–1871
Shreyash N et al. (2021) Green synthesis of nanoparticles and their biomedical applications: a review. ACS Appl Nano Mater 4(11):11428–11457
Chinthala M et al. (2021) Synthesis and applications of nano-MgO and composites for medicine, energy, and environmental remediation: a review. Environ Chem Lett 19(6):4415–4454
Salem SS, Fouda A (2021) Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res 199(1):344–370
Domingues C et al. (2022) Where is nano today and where is it headed? A review of nanomedicine and the dilemma of nanotoxicology. ACS nano 16(7):9994–10041
Kelele KG et al. (2021) Synthesis and characterizations of metal ions doped barium strontium titanate (BST) nanomaterials for photocatalytic and electrical applications: A mini-review. Int J Mater Res 112(8):665–677
Novio F et al. (2013) Coordination polymer nanoparticles in medicine. Coord Chem Rev 257(19-20):2839–2847
Duan H, Wang D, Li Y (2015) Green chemistry for nanoparticle synthesis. Chem Soc Rev 44(16):5778–5792
Bisht N, Phalswal P, Khanna PK (2022) Selenium nanoparticles: A review on synthesis and biomedical applications. Mater Adv 3(3):1415–1431
Cuong HN et al. (2022) New frontiers in the plant extract mediated biosynthesis of copper oxide (CuO) nanoparticles and their potential applications: A review. Environ Res 203:111858
Gawande MB et al. (2016) Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev 116(6):3722–3811
Chakraborty N et al. (2022) Green synthesis of copper/copper oxide nanoparticles and their applications: a review. Green Chem Lett Rev 15(1):187–215
Mishra SR, Ahmaruzzaman M (2022) CuO and CuO-based nanocomposites: Synthesis and applications in environment and energy. Sustain Mater Technol 33:e00463
Marzban A et al. (2022) Biogenesis of copper nanoparticles assisted with seaweed polysaccharide with antibacterial and antibiofilm properties against methicillin-resistant Staphylococcus aureus. J Drug Deliv Sci Technol 74:103499
Ghotekar S et al. (2021) Plant-based green synthesis and applications of cuprous oxide nanoparticles, In: Handbook of Greener Synthesis of Nanomaterials and Compounds, Elsevier, United Kingdom, 201–208
Mao P-H et al. (2022) Single-step synthesized functionalized copper carboxylate framework meshes as hierarchical catalysts for enhanced reduction of nitrogen-containing phenolic contaminants. Catalysts 12(7):765
Chauhan A et al. (2022) Fabrication of copper oxide nanoparticles via microwave and green approaches and their antimicrobial potential. Chem Pap 76(11):7147–7162
Dhatwalia J et al. (2023) Rubus ellipticus fruits extract-mediated cuprous oxide nanoparticles: in vitro antioxidant, antimicrobial, and toxicity study. Chem Pap 77:1377–1393
Akhavan O, Ghaderi E (2011) Copper oxide nanoflakes as highly sensitive and fast response self-sterilizing biosensors. J Mater Chem 21(34):12935–12940
Yang Q et al. (2017) Cuprous oxide nanoparticles trigger ER stress-induced apoptosis by regulating copper trafficking and overcoming resistance to sunitinib therapy in renal cancer. Biomaterials 146:72–85
Akhavan O, Ghaderi E (2010) Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf Coat Technol 205(1):219–223
Fuku X, Modibedi M, Mathe M (2020) Green synthesis of Cu/Cu2O/CuO nanostructures and the analysis of their electrochemical properties. SN Appl Sci 2(5):1–15
Lin L-Y et al. (2018) A highly sensitive non-enzymatic glucose sensor based on Cu/Cu2O/CuO ternary composite hollow spheres prepared in a furnace aerosol reactor. Sens Actuators B: Chem 259:745–752
Hajimammadov R et al. (2018) Random networks of core-shell-like Cu-Cu2O/CuO nanowires as surface plasmon resonance-enhanced sensors. Sci Rep. 8(1):1–8
Khan SR et al. (2020) Investigation of catalytic and fuel additive applications of copper/copper (I) oxide/copper (II) oxide (Cu/CuO/Cu2O) microspheres synthesized by hydrothermal method using sucrose as template. Mater Res Express 7(2):025036
Abd Elkodous M et al. (2022) Facile One-pot Preparation of Cu/CuO/Cu2O Heterojunction for Photocatalytic Applications. Mater Lett 323:132606
Zhao Y et al. (2015) Epitaxial growth of hyperbranched Cu/Cu2O/CuO core-shell nanowire heterostructures for lithium-ion batteries. Nano Res 8(8):2763–2776
Li R et al. (2020) Halides-assisted electrochemical synthesis of Cu/Cu2O/CuO core-shell electrocatalyst for oxygen evolution reaction. J Power Sources 457:228058
Djamila B et al. (2022) In vitro antioxidant activities of copper mixed oxide (CuO/Cu2O) nanoparticles produced from the leaves of Phoenix dactylifera L. Biomass Conversion Biorefinery 1–14
Serra A et al. (2021) Facile cost-effective fabrication of Cu@ Cu2O@ CuO–microalgae photocatalyst with enhanced visible light degradation of tetracycline. Chem Eng J 413:127477
Sahai A et al. (2016) Cu/Cu2O/CuO nanoparticles: Novel synthesis by exploding wire technique and extensive characterization. Appl Surf Sci 390:974–983
Li H et al. (2017) Free-standing and flexible Cu/Cu2O/CuO heterojunction net: a novel material as cost-effective and easily recycled visible-light photocatalyst. Appl Catal B: Environ 207:134–142
Akhavan O, Tohidi H, Moshfegh A (2009) Synthesis and electrochromic study of sol–gel cuprous oxide nanoparticles accumulated on silica thin film. Thin Solid Films 517(24):6700–6706
Collins J, Kalantari S, Post A (1982) Peanut hull flour as dietary fiber in wheat bread. J Food Sci 47(6):1899–1902
Hill GM (2002) Peanut by-products fed to cattle. Vet Clinics: Food Anim Pract 18(2):295–315
Fang Z-F et al. (2014) Cationic surfactant-assisted microwave-NaOH pretreatment for enhancing enzymatic hydrolysis and fermentable sugar yield from peanut shells. BioResources 9(1):1290–1302
Jones G et al. (1998) Development and characterization of paper products from dried sweetpotato stems, peanut shells and soybean pods. SAE transactions 107:316–322
Batalla L, Nunez AJ, Marcovich NE (2005) Particleboards from peanut‐shell flour. J Appl Polym Sci 97(3):916–923
Yallappa S et al. (2017) Natural biowaste of Groundnut shell derived nano carbons: Synthesis, characterization and itsin vitro antibacterial activity. Nano-Struct Nano-Objects 12:84–90
Duc PA et al. (2019) Groundnut shell-a beneficial bio-waste. Biocatalysis and Agricultural. Biotechnology 20:101206
Barwant M et al. (2022) Eco-friendly synthesis and characterizations of Ag/AgO/Ag2O nanoparticles using leaf extracts of Solanum elaeagnifolium for antioxidant, anticancer, and DNA cleavage activities. Chem Pap 76(7):4309–4321
Mahmood RI et al. (2022) Biosynthesis of copper oxide nanoparticles mediated Annona muricata as cytotoxic and apoptosis inducer factor in breast cancer cell lines. Sci Rep 12(1):1–10
Yugandhar P et al. (2017) Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl Nanosci 7(7):417–427
Dubale AA et al. (2015) Heterostructured Cu2O/CuO decorated with nickel as a highly efficient photocathode for photoelectrochemical water reduction. J Mater Chem A 3(23):12482–12499
Holzwarth U, Gibson N (2011) The Scherrer equation versus the’Debye-Scherrer equation’. Nat Nanotechnol 6(9):534–534
Bayuo J, Pelig-Ba KB, Abukari MA (2019) Optimization of adsorption parameters for effective removal of lead (II) from aqueous solution. Phys Chem Indian J 14(1):1–25
Sim SF et al. (2012) Computer-assisted analysis of fourier transform infrared (FTIR) spectra for characterization of various treated and untreated agriculture biomass. BioResources 7(4):5367–5380
Ethiraj AS, Kang DJ (2012) Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res Lett 7(1):1–5
Minh TT et al. (2019) Synthesis of porous octahedral ZnO/CuO composites from Zn/Cu-based MOF-199 and their applications in visible-light-driven photocatalytic degradation of dyes. J Nanomater 2019:1–16
Zadeh FA et al. (2022) Cytotoxicity evaluation of environmentally friendly synthesis Copper/Zinc bimetallic nanoparticles on MCF-7 cancer cells. Rendiconti Lincei. Scienze Fisiche e Naturali 33(2):441–447
Rajagopal G et al. (2021) Mixed phytochemicals mediated synthesis of copper nanoparticles for anticancer and larvicidal applications. Heliyon 7(6):e07360
Biresaw SS, Taneja P (2022) Copper nanoparticles green synthesis and characterization as anticancer potential in breast cancer cells (MCF7) derived from Prunus nepalensis phytochemicals. Mater Today: Proc 49:3501–3509
Cao Y et al. (2021) Green synthesis of bimetallic ZnO–CuO nanoparticles and their cytotoxicity properties. Sci Rep 11(1):1–8
Jinu U et al. (2017) Green engineered biomolecule-capped silver and copper nanohybrids using Prosopis cineraria leaf extract: enhanced antibacterial activity against microbial pathogens of public health relevance and cytotoxicity on human breast cancer cells (MCF-7). Microb Pathogenesis 105:86–95
Chinnaraj S et al. (2022) Silver nanoparticle production mediated by Goniothalamus wightii extract: characterization and their potential biological applications. Particulate Sci Technol 1–15
Azeeze MSTA et al. (2021) Biologically Synthesized Plant-Derived Nanomedicines and Their In vitro–In vivo Toxicity Studies in Various Cancer Therapeutics: Regulatory Perspectives. Cancer Nanotheranostics 2:217–260
Akhavan O, Ghaderi E (2010) Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4(10):5731–5736
Dutta T et al. (2015) ROS generation by reduced graphene oxide (rGO) induced by visible light showing antibacterial activity: comparison with graphene oxide (GO). RSC Adv 5(98):80192–80195
Lakshmi Prasanna V, Vijayaraghavan R (2015) Insight into the mechanism of antibacterial activity of ZnO: surface defects mediated reactive oxygen species even in the dark. Langmuir 31(33):9155–9162
Hashemi E et al. (2014) Cyto and genotoxicities of graphene oxide and reduced graphene oxide sheets on spermatozoa. RSC Adv 4(52):27213–27223
Liu S et al. (2011) Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS nano 5(9):6971–6980
Akhavan O, Ghaderi E, Akhavan A (2012) Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials 33(32):8017–8025
Wang Y-W et al. (2014) Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl Mater Interfaces 6(4):2791–2798
Jannesari M et al. (2020) Graphene/CuO2 nanoshuttles with controllable release of oxygen nanobubbles promoting interruption of bacterial respiration. ACS Appl Mater Interfaces 12(32):35813–35825
Akhavan O et al. (2016) Influence of heavy nanocrystals on spermatozoa and fertility of mammals. Mater Sci Eng: C 69:52–59
Flieger J et al. (2021) Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 14(15):4135
Gulbagca F et al. (2021) Green synthesis of palladium nanoparticles: Preparation, characterization, and investigation of antioxidant, antimicrobial, anticancer, and DNA cleavage activities. Appl Organomet Chem 35(8):e6272
Jadhav MS et al. (2018) Green biosynthesis of CuO & Ag–CuO nanoparticles from Malus domestica leaf extract and evaluation of antibacterial, antioxidant and DNA cleavage activities. N. J Chem 42(1):204–213
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Research involving humans and animals statement
No humans/animals were used for the experiments in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shinde, S., Parjane, S., Turakane, H. et al. Bio-inspired synthesis and characterizations of groundnut shells-mediated Cu/CuO/Cu2O nanoparticles for anticancer, antioxidant, and DNA damage activities. J Sol-Gel Sci Technol 106, 737–747 (2023). https://doi.org/10.1007/s10971-023-06109-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06109-7