Abstract
Biogenic nanoarchitectured magnetic materials have drawn serious attention throughout the last decade. We have attempted the Helleborus niger flower extract functionalized and templated biogenic synthesis of Cu nanoparticles supported Fe3O4 as a likewise novel material. The plant phytomolecules were deployed as a non-toxic sustainable reductant and an outstanding capping agent to stabilize the synthesized NPs. The synthesized Cu/H.niger@Fe3O4 nanocomposite was undergone comprehensive characterizations through Fourier transformed infrared spectroscopy (FT-IR), electron microscopy (SEM and TEM), energy dispersive X-ray spectroscopy (EDX), elemental mapping, vibrating sample magnetometer (VSM), X-ray diffraction (XRD) and inductively coupled plasma (ICP) techniques. The material was catalytically explored in the synthesis of diverse pyrano[3,2-c]chromene derivatives by coupling 4-hydroxycoumarin, malononitrile and a range of aldehydes in hot water when it afforded excellent yields. Based on its core magnetism, the catalyst was easily recovered using a magnet and reused for 8 successive times without considerable loss in catalytic activity. After the chemical application, the synthesized Cu/H.niger@Fe3O4 nanocomposite was engaged in biological assays like study of anti-oxidant properties by DPPH mediated free radical scavenging test using BHT as a reference molecule. Thereafter, on having a significant IC50 value in radical scavenging assay, we extended the bio-application of the desired nanocomposite in anticancer study of A549 and H358 human lung cell lines in-vitro through MTT assay. The cell viability of malignant lung cell line reduced dose-dependently in the presence of desired nanocomposite. So, these results suggest that synthesized Cu/H.niger@Fe3O4 as a chemotherapeutic nanomaterial have a suitable anticancer activity against lung cell lines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the last few years advanced functional nanomaterials have got tremendous impetus in applied material research due to their distinctive physicochemical and biochemical characteristics [1,2,3]. In particular, the noble metal nanoparticles (NPs) like Cu, Ag, Au, Pd, Pt etc. have been the top-notch of preference among a great deal of researchers due to their wide perspective of implications. They are literally noble because of their enhanced stability, resistance towards aerial oxidation in moist air or corrosion during catalysis, biocompatibility, less cytotoxicity towards bioapplications, dependence of properties based on size, shape, state of aggregation and local environment etc. [4,5,6]. Despite the use of various efficient physical and chemical protocols for the controlled synthesis of these NPs, green and sustainable syntheses always have been of top priority. The conventional methods frequently use high temperature, harsh reducing agents, high pressure, carcinogenic solvents and costly chemicals or equipments. As a substitute, green approach in the synthesis of NPs have come into great prominence employing diverse biopolymers, different microorganisms like bacteria, yeasts, fungi, algae and phytomolecular contents derived from plants [7,8,9,10]. Some of the advantages of using such methods include environmental compatibility, eco-friendly conditions, slow rate of crystal growth, controlled and uniform particles shape and size, high dispersion of particles in the reaction medium, simple conditions to scale up and high thermo-mechanical stability [11, 12].
However, the concept of bio-inspired synthesis utilizing different plant extracts like leaves, flower, fruit, bark, seed, root, latex, gum etc. has been more beneficial in some special aspects [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. In this process there is negligible probability of chemical or microbial contamination which is extremely unfavorable in biomedical applications. The electron rich environment provided by the adorned phytomolecules facilitates the sustainable reduction of immobilized noble metal ions into related NPs. In addition, the phytomolecular capping makes these biogenic metal NPs amazingly stable [28,29,30,31].
Following with the trend, we are prompted to report herein, the Helleborus niger flower extract mediated biogenic synthesis of Cu NP doped Fe3O4, a hybrid bio-nanocomposite material (Cu/H.niger@Fe3O4). It was subsequently utilized in the catalytic synthesis of pyrano[3,2-c]pyrazine derivatives by atom-economical multicomponent coupling and also towards in the bio-application of inhibiting the proliferation of human lung cancer cells.
Cu NPs has been the most familiar among the other noble metals due to its abundance, inexpensiveness, less toxicity, high biocompatibility, stability, and electrical conductivity. It has been reported for the diverse uses due to its significant electrical, optical, catalytic, biomedical, antifungal, and antibacterial properties [32]. One of the major drawbacks in the conventional physical and chemical methods is their propensity towards oxidation to Cu2O or CuO [33]. In our protocol, we have exploited the plant supported synthesis under milder conditions that stabilizes the NPs by phytomolecular encapsulation. Helleborus niger is a perennial herb grown abundantly in Asia and Europe and commonly used as traditional medicine in many countries. Phytochemical research revealed that the herb is rich in different biomolecules like alkaloids, terpenoids, glycosides and saponosides, which contains a large number of oxygen functions [34, 35] (Fig. 1). The tendency of Cu NPs to self-aggregate could be suppressed by dispersing them uniformly over Fe3O4 NPs. The ferrite attachment has the advantage of facilitating surface phyto-functionalization utilizing its hydroxyl groups and also the easy magnetic separation from the system [36,37,38].
Subsequently, the Cu/H.niger@Fe3O4 nanocomposite was subjected to heterogeneous catalysis in the atom-economical three component condensation of 4-hydroxycoumarin, malononitrile and different aromatic aldehydes to diverse pyrano[3,2-c]chromene derivatives, a biologically potent heterocycle. The moiety is reported to build the structural backbone of different naturally occurring compounds and exhibits numerous therapeutic properties [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. We followed a very simple procedure by assembling the three components in refluxing water when the material efficiently catalyzed it to afford the product in excellent yields. After the chemical evaluation, we wished to exploit the Cu/H.niger@Fe3O4 material in biological assessments towards controlling the growth of lung cancer cells in human body. Cytotoxicity of the nanocomposite was analyzed over two standard lung cancer lines, A549 and H358 through the recognized MTT assay. Lung cancer is considered as one of deadliest category of carcinoma, affecting both men and women and is responsible for a great deal of mortality. In recent times, different bionanomaterials have been employed as effective chemotheraputic nanodrugs, treating cancer in an unconventional formulation [54,55,56,57]. This has inspired us to use our devised novel nanocomposite material in the treatment of lung cancer following in vitro studies. Notably, in our research we achieved some excellent outcomes against the lung cancer cell lines that could lead the material in future towards a high end prospect in lung cancer studies.
2 Experimental
2.1 The Extraction of Helleborus niger Flower
2.0 g of the H. niger dried flower was dispersed over 50 mL DI H2O and gently warmed to 80 °C for half an hour. The yellow solution was filtered over Whatman-1 paper to remove the undissolved materials. The filtrate was considered as flower extract, being preserved at 4 °C for further study.
2.2 Green Synthesis of the Cu/H.niger@Fe3O4 Nanocomposite
Fe3O4 NPs were synthesized following a well-reported co-precipitation method [57]. 0.5 g of this NP was dispersed by sonication in 100 mL DI H2O for 20 min. In order to impregnate Cu2+ ions over the ferrite NPs 0.025 g Cu(NO3)2.3H2O was stirred over it for 1 h. Subsequently, the plant extract was introduced and stirred for 5 h at 100 °C when the phytomolecules sustainably reduced the ions into Cu NPs. The Cu/H.niger@Fe3O4 nanocomposite was finally recovered using a bar magnet, rinsed with DI H2O and dried at 60 °C in vacuum oven. By ICP method the Cu content was found as 0.11 mmol g−1.
2.3 Typical Synthesis of Pyrano[3,2-c]chromenes
4-hydroxycoumarin, aromatic aldehyde, malononitrile (equimolar) and Cu/H.niger@Fe3O4 catalyst (25 mg) were mixed together and refluxed in DI water (5 mL) over. After completion (by TLC), the catalyst was removed magnetically and the crude product was filtered out followed by purification via recrystallization from refluxing ethanol.
2.4 Antioxidant Analysis
While determining the antioxidant properties of Cu/H.niger@Fe3O4 nanomaterial, the DPPH (1,1-diphenyl-2-picrylhydrazil) radical scavenging analysis was carried out. In the protocol, different concentration of the probe sample was mixed to the methanolic solution of DPPH radical. Now, the DPPH purple colored solution after abstracting the protons or free electrons from experimental antioxidant sample, got quenched and converted into yellow color. Antioxidant competence of the material is comparative to the intensity of yellow color of quenched DPPH solution, being determined spectrophotometrically.
2.5 Study of Cytotoxicity
Cytotoxicity of the Cu/H.niger@Fe3O4 nanocomposite was evaluated over the A549 and H358 lung cancer cell lines through the well-recognized MTT analysis. In the beginning, the cells were methodically cultured in 96-welled plates having 1 × 105 cell/well inside a humidified incubator maintaining standard conditions (37 °C, 5% CO2 atmosphere) for 24 h. When the cell confluence was about 80% in a monolayer, the culture media (10% FBS) was removed followed by washing with PBS for twice. The Cu/H.niger@Fe3O4 nanocomposite were prepared in 5 variable concentrations (0.5–1000 µg/mL) in RPMI medium and brought into the developed cells. The mixtures were incubated again for 72 h at the same conditions and then 10 μL solution of MTT dye in PBS (5 mg/mL) was mixed to each of them. They were incubated once again for 4 h, cell media was made with 100µL DMSO and shaken to facilitate the formazan crystal solubilization. Finally, absorption intensity of the colored complexes were measured at 545 nm equipped with an ELISA microplate reader.
3 Results and Discussion
3.1 Analysis of Characterization Data for Cu/H.niger@Fe3O4 Nanocomposite
Herein, we have demonstrated an efficient biogenic method for the synthesis of Cu NP fabricated Fe3O4 NP being templated over H. niger extract. The plant extract contained several oxygenated phytochemicals that have the active role to reduce the immobilized metal ions towards the formation of corresponding NPs. Such bio-inspired synthesis involves two successive steps, adsorption of Cu2+ ions over the ferrite NPS followed by the in situ green reduction of those ions avoiding any toxic and unsafe reducing agents. The phyto-molecules additionally stabilize the synthesized NPS by capping. This also facilitates the NPs to keep apart from each other thereby minimizing the chance of self aggregation (Scheme 1). The physicochemical features of the as-synthesized material were assessed by a variety of instrumental methods like FT-IR, SEM, EDX, TEM, elemental mapping, ICP, VSM and XRD.
Figure 2 describes the FT-IR spectroscopic analysis of Cu/H.niger@Fe3O4 nanocomposite. Fe3O4 NP is acknowledged by the characteristic signals found at 446 and 584 cm−1, related to the octahedral bending and tetrahedral stretching vibrations of Fe–O–Fe bond. The peak observed at 631 cm−1 corroborates to the spinel structure of ferrite. The O–H stretching and bending vibrations are related to the vibrational peaks found at 3429 and 1653 cm−1 associated with surface hydroxyl groups over Fe3O4 as well as the intercalated water respectively. The plant biomolecular attachment was confirmed by a group of vibrational bands detected at 3429, 2922, 1739, 1624, 1462 and 1053 cm−1, ascribed to O–H stretching (merged with ferrite hydroxyls), C–H, C=O, C=C, C–O and C–O–C stretching vibrations respectively, justifying the occurrence of polyols, flavonoids, tannins, catechins, alkaloids and terpenoids in the plant extract.
The surface morphology, particle shape and size, and the relative dispersion of Cu/H.niger@Fe3O4 nanocomposite was determined by Electron microscopy (TEM and SEM) (Figs. 3 and 4). Figure 3a represents the unmodified Fe3O4 NPs which are uniformly spherical of 10–15 nm dimensions. The particles are well distributed without any prominent agglomeration. Another image of the nanocomposite (Fig. 3b) shows a clear distribution of the two different kind of NPs, grey and black colored particles signifying Fe3O4 and Cu NP respectively. Cu NPs seems to be of larger sized (25–30 nm) than ferrite NPs, spread over the latter. The shade like appearance is supposed to be due to surface modification by H. niger phytochemicals over the Cu–Fe3O4 nanocomposite. The large surface area of this composite was utilized in immobilizing the plant biomolecules which facilitated the green reduction of copper ions into NPs. SEM image also authenticates the globule shaped nanocomposite. However, individual identification of the two patterns of NPs could not be possible from SEM analysis. It is due to manual sampling the material looks agglomerated (Fig. 4), although TEM analysis revealed the exact scenario.
After the electron microscopic study, we had EDX analysis, equipped with the SEM instrument, in order to know its chemical constitution. Figure 5 depicts the corresponding spectrum of Cu/H.niger@Fe3O4 nanocomposite. It evidently says the occurrence of Fe and Cu as major metallic component in it, while C and O as non-metals. The broad significant signal of Au is found at 2.1 keV, endorsed to the Au vapor deposition over experimental sample prior to analysis. The presence of phytomolecules over the nanocomposite can be validated by the non-metallic signals in the EDX profile. The EDX outcomes were additionally concreted through elemental mapping study. We could have a fine knowledge of the atomic dispersion of the constituent elements by this study. Figure 6 is an outcome of the X-ray scanning of a section of the SEM image where the elemental species are in the form of dots, being homogeneously spread over the matrix. The uniform dispersion certainly has superior effect on its catalytic activity.
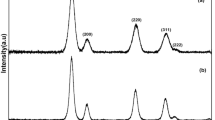
The crystalline behavior and nature of phases of Cu/H. niger@Fe3O4 nanocomposite was resolved by XRD investigation, displayed in Fig. 7. The sharp diffraction peaks of Fe3O4 are quite evident from the signals observed at 2θ = 31.1, 36.4, 44.3, 54.7, 58.3, 64.1° which finds close resemblances to JCPDS standard 19-0629, correlated to diffraction on (220), (311), (400), (422), (511) and (440) Fe3O4 crystal planes. On the other hand, the weak diffraction peaks related to Cu NPs appeared at 42.7, 49.7 and 71.8 are attributed to the (111), (200) and (220) planes, respectively. The poorly crystalline region below the 20o diffraction angle characterizes the biomolecular attachment.
The study of magnetic properties following VSM analysis for an iron cored material like Cu/H. niger@Fe3O4 nanocomposite, is a ubiquitous task. The corresponding outcome in the form of a magnetic hysteresis curve is revealed in Fig. 8. The corresponding magnetization saturation (Ms) value obtained was 58.4 emu/g, validating the material as superparamagnetic in nature and easy magnetically retrievable.
3.2 The Catalytic Exploration of Cu/H.niger@Fe3O4
After the meticulous study on physicochemical characterizations of Cu/H.niger@Fe3O4 nanocomposite, the next endeavor was to investigate its catalytic properties. In so doing, we subjected the material as a magnetically reusable nanocatalyst by coupling 4-hydroxycoumarin, malononitrile and diverse aryl aldehyde towards a range of 2-amino-4-aryl-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile compounds (Scheme 1). However, prior to generalization, optimization of reaction conditions appeared necessary and accordingly a model reaction was chosen to test the effect of diverse constraints such as solvent, catalyst load and temperature. Table 1 shows the condensation of three components using benzaldehyde over Cu/H. niger@Fe3O4 catalyst. To maintain the green chemistry criteria we always have the priority to use water as the solvent and it afforded 95% yields in just 3 h. However, we also tried other solvents like EtOH, hexane, CH3Cl, CH2Cl2, toluene, acetonitrile (entries 1–7) but none of them were better than water (Entry 7). Again, maintaining the best solvent we varied the amount of catalyst amount keeping the best solvent and 25 mg was found optimum (entries 7–9). In the temperature variation study, there was no considerable yield at lower temperatures (entry 10) and the best productivity was found at 100 °C. Markedly, there was only a moderate yield under catalyst free condition (entry 11). Consequently, the best condition was achieved with equimolar reagents under refluxing water (5 mL) in presence of 25 mg of Cu/H. niger@Fe3O4 nanocatalyst.

Next, we considered to check the validity of these optimized conditions by varying the substrates with different aromatic and heteroaromatic aldehydes. Table 2 documents the corresponding outcomes where the different functionalities were highly compatible under the optimized reaction conditions irrespective of their electronic characteristics (donating or withdrawing) and geometric positions (ortho/meta/para). In all the cases very good to excellent yield were obtained (entries 1–11). In addition to benzenoid aromatics, the heteroaromatic compound 2-furfural also responded very well in the reaction (entry 12).
3.3 Resuability Studies
Since the catalyst contained magnetic cored ferrite material, it was quite easy for the nanocomposite catalyst from the system, merely using an external magnet. After completion of a fresh batch of model reaction, the catalyst was recovered magnetically and washed with aqueous ethanol for twice. It was subsequently dried at 60 °C for 3 h under vacuum for regeneration. The material was sufficiently robust for reusing it for successive 8 times without significant decrease in its activity (Fig. 9).
3.4 Antioxidant Assay of Cu/H.niger@Fe3O4 Nanocomposite
It is reported that a material having high antioxidant potency, would have significant cancer proliferation and apoptosis ability. Thereby, before projecting the Cu/H.niger@Fe3O4 nanocomposite material in cancer studies, we felt urge to investigate its antioxidant efficiency. The very familiar DPPH radical scavenging study was accomplished in this regard. The material was added to DPPH methanolic solution at six variable concentrations, starting from 31.25 to 1000 μg/mL and evaluated using BHT as the reference molecule. On scavenging electrons from the experimental samples the DPPH free radical gets quenched to a stable molecular species which converts the purple colored solution into pale yellow. The scavenging capacity is determined through % inhibition, as measured spectrophotometrically (UV–Vis spectroscopy) at 517 nm followed by using Eq. (1). Obviously, the scavenging capacity got enhanced with higher concentrations and became highest at 1000 μg/mL (Fig. 10).
3.5 Cytotoxicity Studies over Cu/H.niger@Fe3O4 Nanocomposite
After having a significant IC50 value in the DPPH radical scavenging antioxidant assay, we further explored the cytotoxicity of Cu/H.niger@Fe3O4 nanocomposite over the two human lung cancer cell lines, A549 and H358 in vitro following MTT method. Mechanism wise it is anticipated that the Cu NPs effectively reduce the ATP content of the cell which causes mitochondrial damage which in turn enhances the generation of reactive oxygen species (ROS) around the Cu NPs. Cytotoxicity is very closely related to the concentration of ROS. In our protocol the processed cells were brought in contact to different concentrations of Cu/H.niger@Fe3O4 nanomaterial (5–2000 µg/mL) and the procedures are followed stated in Sect. 2.6. Noticeably, % cell viability decreased dose dependently of the catalyst over both the cell lines (Figs. 11, 12). The corresponding IC50 values over the two cell lines were 129.58 and 193.26 μg/mL respectively. The significant outcomes would certainly validate the Cu/H.niger@Fe3O4 material as an efficient chemotherapeutic nano-drug against human lung cancer, in vitro.
4 Conclusion
In summary, the successful synthesis of a novel biodegradable, bio-inspired magnetic Cu-Fe3O4 nanocomposite has been stated here. H. niger flower extract has been exploited for the green and biogenic reduction of immobilized Cu ions over the surface of pre-synthesized Fe3O4 NPs. The phytomolecular capping over the tiny Cu NPs made them considerably stable. The sustainable material was meticulously characterized over a great number of analytical techniques. In catalytic exploration, the Cu/H.niger@Fe3O4 nanocomposite was found highly effective in the three component atom-efficient synthesis of miscellaneous pyrano[3,2-c]chromene derivatives by coupling 4-hydroxycoumarin, malononitrile and different aldehydes in refluxing water affording high yields. The catalyst was then retrieved magnetically and semi-pure crude products were collected by filtration and then finally purified by recrystallization. The magnetic nanocatalyst was easily recovered, washed with aqueous EtOH and dried for regeneration in order to reuse. In the biological evaluation the Cu/H.niger@Fe3O4 nanocomposite was first explored as antioxidant agent through DPPH radical scavenging assay. It exhibited nearly 95% activity at a dose of 1000 µg/mL. Thereafter the material was used in inhibiting human lung cancer cells by studying cytotoxicity over A549 and H358 cell lines with standard MTT assay. Here too the % cell viability got reduced dose-dependently over the both cell lines and the corresponding IC50 values were observed to be 128.35 and 196.29 μg/mL respectively.
References
C. Xu, Y.S. Zhang, S. Begin, N.T.K. Thanh, Nanoscale (2022). https://doi.org/10.1039/D2NR90077G
A.G. Leonel, A.A.P. Mansur, H.S. Mansur, Water Res. 190, 116693 (2021)
D. Sharma, S. Kanchi, K. Bisetty, Arab. J. Chem. 12, 3576–3600 (2019)
G. Vinci, M. Rapa, Bioengineering 6, 10 (2019)
G. Habibullah, J. Viktorova, T. Ruml, Nanoscale Res. Lett. 16, 47 (2021)
Y. Vladimis, V. Voliani, Front. Bioeng. Biotechnol. 6, 143 (2018)
M. Kowshik, S. Ashtaputre, S. Kharrazi, W. Vogel, J. Urban, S.K. Kulkarni et al., Nanotechnology 14, 95 (2003)
S. Ahmed, M. Ahmad, B.L. Swami, S. Ikram, J. Adv. Res. 7, 17–28 (2016)
X.F. Zhang, Z.G. Liu, W. Shen, S. Gurunathan, Int. J. Mol. Sci. 17, E1534 (2016)
P. Mohanpuria, N.K. Rana, S.K. Yadav, J. Nanopart. Res. 10, 507–517 (2008)
M. Rai, A. Ingle, Appl. Microbiol. Biotechnol. 94, 287–293 (2012)
M.S. Akhtar, J. Panwar, Y.S. Sun, ACS Sustain. Chem. Eng. 6, 591–602 (2013)
P.S. Vankar, D. Shukla, Appl. Nanosci. 2, 163–168 (2012)
D. Philip, C. Unni, S.A. Aromal, V.K. Vidhu, Spectrochim. Acta A 78(2), 899–904 (2011)
T. Santhoshkumar, A.A. Rahuman, G. Rajakumar, S. Marimuthu, A. Bagavan, C. Jayaseelan, A.A. Zahir, G. Elango, C. Kamaraj, Parasitol. Res. 108(3), 693–702 (2011)
M.R. Bindhu, M. Umadevi, Spectrochim. Acta A 101, 184–190 (2013)
H. Veisi, S. Azizi, P. Mohammadi, J Clean. Prod. 170, 1536–1543 (2018)
V.K.M. Katta, R.S. Dubey, Mater. Today Proc. 45, 794–798 (2021)
M. Kandiah, K.N. Chandrasekharan, J. Nanotechnol. 5512786, 1–18 (2021)
B. Ankamwar, C. Damle, A. Ahmad, M. Sastry, J. Nanosci. Nanotechnol. 5, 1665–1671 (2005)
M.M.I. Masum, M.M. Siddiqa, K.A. Ali, Y. Zhang, Y. Abdallah, E. Ibrahim, W. Qiu, C. Yan, B. Li, Front. Microbiol. 10, 820 (2019)
A.R. Vartooni, M. Nasrollahzadeh, M. Alizadeh, J. Colloid Interface Sci. 470, 268–275 (2016)
A. Rautela, J. Rani, M. Debnath, J. Anal. Sci. Technol. 10(5), 1–10 (2019)
T.Y. Suman, S.R. Radhika Rajasree, A. Kanchana, S.B. Elizabeth, Colloids Surf. B 106, 74–78 (2013)
I. Ilahi, F. Khuda, M.U.K. Sahibzada, S. Alghamdi, R. Ullah et al., Arab. J. Chem. 14, 103110 (2021)
M. Nakhjavani, M. Mohsen Sarafraz, V. Nikkhah, S. Shoja, M. Sarafraz, Heat Mass Transfer 53, 3201–3209 (2017)
H.P. Borase, C.D. Patil, R.B. Salunkhe, R.K. Suryawanshi, B.K. Salunke, S.V. Patil, Bioprocess Biosyst. Eng. 37, 1695–1717 (2014)
N. Durán, P.D. Marcato, M. Durán, A. Yadav, A. Gade, M. Rai, Appl. Microbiol. Biotechnol. 90, 1609–1624 (2011)
D. Ballotin, S. Fulaz, M.L. Souza, P. Corio, A.G. Rodrigues, A.O. Souza et al., Nanoscale Res. Lett. 11, 313 (2016)
P. Azmath, S. Baker, D. Rakshith, S. Satish, Saudi Pharm. J. 24, 140–146 (2016)
Y. Cai, B. Karmakar, H.S. Alsalem, A.F. El-kott, M.Z. Bani-Fwaz et al., Arab. J. Chem. 15, 103848 (2022)
M.B. Gawande, A. Goswami, F.-X. Felpin, T. Asefa, X. Huang, S. Silva, X. Zou, R. Zboril, R.S. Varma, Chem. Rev. 116(6), 3722–3811 (2016)
P. Singh, K. Kumari, V.K. Visvakarma, G.K. Mehrotra, R. Chandra, D. Kumar, R. Patel, V.V. Shahare, Green Technol. Environ. Sustain. 1866, 309–337 (2017)
V. Kishor Kumar, K.G. Lalitha, Anc. Sci. Life 36, 151–158 (2017)
M.C. Maior, C. Dobrata, Cent. Eur. J. Biol. 8, 272–285 (2013)
W. Xue, G. Yang, B. Karmakar, Y. Gao, Arab. J. Chem. 14, 103306 (2021)
C. Wang, B. Karmakar, N.S. Awwad, H.A. Ibrahium, A.F. El-kott, M.M. Abdel-Daim, A.A.A. Oyouni, O. Al-Amer, G.E. Batiha, Arab. J. Chem. 15, 103809 (2022)
X. Ou, B. Karmakar, N.S. Awwad, H.A. Ibrahium, H.H. Osman, A.F. El-kott, M.M. Abdel-Daim, Inorg. Chem. Commun. 137, 109221 (2022)
H. Gourdeau, L. Leblond, B. Hamelin, C. Desputeau, K. Dong, I. Kianicka, D. Custeau, C. Boudreau, L. Geerts, S.-X. Cai et al., Mol. Cancer Ther. 3, 1375–1384 (2004)
B.S. Chetan, M.S. Nimesh, P.P. Manish, G.P. Ranjan, J. Serb. Chem. Soc. 77, 1–17 (2012)
A. Burgard, H.J. Lang, U. Gerlach, Tetrahedron 55, 7555–7562 (1999)
J.M. Evans, C.S. Fake, T.C. Hamilton, R.H. Poyser, G.A. Showell, J. Med. Chem. 27, 1127–1131 (1984)
R.-R. Zhang, J. Liu, Y. Zhang, M.-Q. Hou, M.-Z. Zhang, F. Zhou, W.-H. Zhang, Eur. J. Med. Chem. 116, 76–83 (2016)
D.C. Mungra, M.P. Patel, D.P. Rajani, R.G. Patel, Eur. J. Med. Chem. 46, 4192–4200 (2011)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517–520 (1993)
A.R. Saundane, K. Vijaykumar, A.V. Vaijinath, Bioorg. Med. Chem. Lett. 23, 1978–1984 (2013)
J.A. Makawana, M.P. Patel, R.G. Patel, ArchivDerPharmazie 345, 314–322 (2012)
A. Venkatesham, R.S. Rao, K. Nagaiah, J.S. Yadav, G.R. Jones, S.J. Basha, B. Sridhar, A. Addlagatta, Med. Chem. Comm. 3, 652–658 (2012)
N. Jagadishbabu, K. Shivashankar, J. Heterocyclic Chem. 54, 1543–1549 (2017)
K. Shivashankar, M.V. Kulkarni, L.A. Shastri, V.P. Rasal, S.V. Rajendra, Phosphorus Sulfur Silicon Relat. Elem. 181, 2187–2200 (2006)
K. Shivashankar, L.A. Shastri, M.V. Kulkarni, V.P. Rasal, S.V. Rajendra, Phosphorus Sulfur Silicon Relat. Elem. 183, 56–68 (2007)
A. Maleki, Z. Varzi, F.-H. Afruzi, Polyhedron 171, 193–202 (2019)
A. Maleki, M. Niksefat, J. Rahimi, R.-T. Ledari, Mater. Today Chem. 13, 110–120 (2019)
Z. Shi, Y. Mahdavian, Y. Mahdavian, S. Mahdigholizad, P. Irani, M. Karimian et al., Arab. J. Chem. 14, 103224 (2021)
Y. Cai, B. Karmakar, H.S. Alsalem, A.F. El-kott, M.Z. Bani-Fwaz, S. Negm, A.A.A. Oyouni, O. Al-Amer, G.E. Batiha, Arab. J. Chem. 15, 103848 (2022)
Y. Huang, Y. Kang, A. El-kott, A.E. Ahmed, A. Khames, M.A. Zein, Arab. J. Chem. 14, 103299 (2021)
Y. Xue, B. Karmakar, J. Ke, H.A. Ibrahium, N.S. Awwad, A.F. El-kott, J. Saud, Chem. Soc. 26, 101391 (2022)
A. Baziar, M. Ghashang, React. Kinet. Mech. Catal. 118, 463–479 (2016)
K. Pradhan, S. Paul, A.R. Das, Catal. Sci. Technol. 4, 822–831 (2014)
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research groups program under Grant Number (R.G.P. 2/76/43). Also, Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R185), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that there is no competing interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, Y., Karmakar, B., AlSalem, H.S. et al. Green Nanoarchitectonics of Cu/Fe3O4 Nanoparticles Using Helleborus niger Extract Towards an Efficient Nanocatalyst, Antioxidant and Anti-lung Cancer Agent. J Inorg Organomet Polym 32, 3585–3594 (2022). https://doi.org/10.1007/s10904-022-02430-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02430-w