Abstract
Nanopowder is frequently employed in the fabrication of miniaturized multilayer devices due to the advantage of small particle size. In this work, sol-gel method was employed for the synthesis of Bax-0.003La0.003TiO3 nanopowders. Abnormal grain growth was observed in the Ba-excessive composition at a relatively low sintering temperature. This result was inconsistent with previous reports that grain growth was suppressed in the Ba-excessive BaTiO3 ceramics. Combining a comparison study, BaCO3, second phase in the powder with x = 1.03, was confirmed to be responsible for the abnormal grain growth. Based on the results of SEM, XRD, TG-DTA and electrical properties, it is reasonable to speculate that BaCO3 and oxygen vacancy formed during reduction sintering together triggered the reactive liquid-phase sintering in the Bax-0.003La0.003TiO3 ceramics with x = 1.03. Thus abnormal grain growth occurred, meanwhile, semiconducting grains was formed.
Graphical Abstract

Highlights
-
Abnormal grain growth was observed in the Ba-excessive La-doped BaTiO3 ceramics prepared from nanopowder synthesized by sol-gel method, when sintered in N2 atmosphere at a relatively low temperature.
-
The BaCO3, as second phase, was found to be responsible for the abnormal grain growth.
-
The BaCO3 and oxygen vacancy formed during reduction sintering together triggered the reactive liquid-phase sintering in the Bax-0.003La0.003TiO3 ceramics with x = 1.03.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Donor-doped barium titanate ceramics can be an n-type semiconductor and exhibit a positive temperature coefficient of resistivity (PTCR) [1,2,3]. They have been widely used in electronic circuits as temperature sensors, current limiters, heating devices and so on because of the excellent PTCR effect [4]. Nowadays electronic elements with small volume are urgently needed due to the demands of miniaturized electronic products. Then the PTCR ceramics are fabricated with a multilayer structure to scale down the volume [5]. The grain size of the laminated PTCR ceramics is requested to be very fine to withstand high electric field in the thin layers [6, 7]. Then, nanopowder is frequently employed and critically desired in the fabrication of miniaturized multilayer devices owing to the advantage of small particle size [8]. Thus, it is important to study the microstructural evolution and electrical properties of the PTCR ceramics prepared from nanopowder.
The techniques such as two-step sintering, spark plasma sintering and so on have been confirmed to be effective to suppress grain growth through reducing sintering times or lowering sintering temperatures [8, 9]. However, high-temperature sintering is usually needed for the formation of semiconducting grains in the donor doped BaTiO3 ceramics [10]. Grain coarsening easily appeared during high-temperature sintering, which is not favored in the multilayer PTCR ceramics [10, 11]. Other parameters, such as sintering aids or defects, etc., may also influence the sintering kinetics [12]. For examples, liquid phases were commonly introduced to improve the electrical properties, which could greatly enhance mass transportation and promote the grain growth [13]. Besides, the multilayer PTCR ceramics should be sintered in reducing atmosphere to prevent the oxidation of inner Ni electrodes [14, 15]. The grain growth can be enhanced by the diffusion of oxygen vacancies formed during reduction sintering [12]. Therefore, the grain-size distribution depends crucially on the composition, sintering condition and so on. Although fine-grain PTCR ceramics have been reported by researchers [6, 8, 16], the results have not been full satisfactory to produce fine-grain ceramics, especially with narrow grain-size distribution. Accordingly, it is important to further discover factors that influence the grain growth of PTCR ceramics prepared from nanopowder.
Ba-excessive composition is favored for the fabrication of multilayer PTCR ceramic, because it usually exhibited fine-grain microstructure and high PTCR effect [10]. In the present work, La-doped BaTiO3 nanopowder was synthesized through sol-gel method. The grain growth and electrical properties were studied in the ceramics sintered at a relatively low temperature in N2 atmosphere. Abnormal grain growth was observed in Ba-excessive ceramics, which was contrary to previous experience. The mechanism for this abnormal phenomenon was further investigated, together with the electrical properties.
2 Experimental details
La-doped BaTiO3 nanopowder with formula Bax-0.003La0.003TiO3 (x = 1.00 and 1.03) was synthesized by the sol-gel method [3, 16], using Ba(CH3COO)2 (>99.0%; Sinopharm, China), La(NO3)3·6H2O (99.9%; Sinopharm, China) and Ti(OC4H9)4 (>98.0%; Sinopharm, China). Details for the synthesis (see Fig. 1) are as follows: Ti(OC4H9)4 was diluted in a mixed ethanol-acetic acid solvent to obtain a solution with Ti4+ concentration of 1.25 mol/L and stoichiometric amount of Ba(CH3COO)2 and La(NO3)3·6H2O were diluted into deionized water. After 30 min of vigorous stirring at room temperature, two clear solutions were obtained. Then the solution containing Ba2+ and La3+ was added into the solution containing Ti4+ dropwise to avoid the rapid hydrolysis of Ti(OC4H9)4. The acquired sol was aged at room temperature for 12 hours and then dried at 90 °C to form gel. The obtained gel was pulverized into powder and calcined at 800 °C in air for 2 hours.
Tape casting method was employed to prepare green pellets as reported previously [17]. The green ceramics were sintered in N2 atmosphere. The sintering was conducted at temperature range from 1075 to 1150 °C for 2 h. The heating and cooling rates were controlled at 300 °C/h. Then the reduced ceramics were reoxidized at 800 °C for 2 h with a heating rate of 300 °C/h.
Densities of the ceramics were measured by the geometrical method. The X-ray diffraction technique (XRD, XRD-7000s, Shimadzu) was used for the phase composition identification of the raw materials and obtained ceramics. Thermogravimetric and differential thermal analysis (TG-DTA, STA 449F3, Netzsch) were conducted in N2 atmosphere at a heating rate of 10 °C/min for the raw materials. Scanning electron microscope (SEM, FEI Nova 630) was employed to investigate the microstructures for the raw materials and obtained ceramics. Resistivity-temperature (R-T) curves were collected using a computer controlled analyzer at temperatures ranged from room temperature to 250 °C. The PTCR effect was defined as the ratio of the resistivity at 250 °C to that at room temperature (RT). Complex impedance spectroscopy was measured by using a WK6550B impedance analyzer combined with a temperature controller (Huace 650 T, Beijing Huace Testing Instrument Co. Ltd., China).
3 Results and discussions
3.1 Analysis for the synthesized Bax-0.003La0.003TiO3 nanopowders
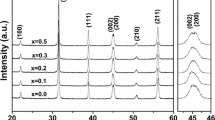
Figure 2 shows the SEM images and XRD patterns of the synthesized powders with a formula of Bax-0.003La0.003TiO3 calcined at 800 °C under air atmosphere. From Fig. 2a, b, a similar microstructure can be observed for the two powders and the particle size estimated was about 60 nm. XRD results shown in Fig. 2c suggested that a perovskite structure with a cubic symmetry was formed. Meanwhile, BaCO3 as second phase was observed in the powder with x = 1.03, which was also reported in previous studies [18]. It should be noted here that the solution of barium element in BaTiO3 lattice is less than 100 ppm [19, 20], so the excessive barium element in the powder with x = 1.03 mainly existed in the form of BaCO3.
3.2 Microstructural evolution and electrical properties
Figure 3 shows the microstructure of the reduced Bax-0.003La0.003TiO3 ceramics sintered at 1100 and 1150 °C, respectively. The grain growth behavior is found to be distinctly different, which was closely dependent on the stoichiometry. At x = 1.00, the ceramics exhibited porous microstructures and homogeneous grain-size distributions. The average grain size increased from 60 nm to about 220 and 270 nm after sintered at 1100 and 1150 °C. On the other hand, dense microstructure was observed in the ceramics with x = 1.03. The average grain size abnormally increased to about 1.6 and 2.1 μm after sintered at 1100 and 1150 °C, respectively. This result was obviously different from previous reports that grain growth was usually suppressed in Ba-excessive BaTiO3 ceramics prepared by solid-state reaction [10, 21].
Figure 4a shows the influence of sintering temperature on the electrical properties of the Bax-0.003La0.003TiO3 ceramics reoxidized at 800 °C. The RT resistivity of the ceramics with x = 1.03 was obviously lower than the ones with x = 1.00, especially at low sintering temperature. For example, at 1075 °C, the ceramic with x = 1.00 was almost insulated and the RT resistance was too high to be measured (> 108 Ω). However, the RT resistivity became evidently lower and a value of only 7.5 × 102 Ωcm was obtained at x = 1.03. The RT resistivity was able to be further decreased at an elevated sintering temperature and the pronounced PTCR effect could be observed as depicted in Fig. 4b.
Impedance analysis was employed to further investigate the electrical properties and the Bax-0.003La0.003TiO3 ceramics sintered at 1075 °C and reoxidized at 800 °C were selected as representatives. Considering that the ceramic with x = 1.00 was high resistance, it was then heated to about 500 °C to lower its resistance and then impedance data was collected [22, 23]. In contrast, the ceramic with x = 1.03 was low-resistivity and the impedance analysis was conducted at room temperature. Both the ceramics exhibited typical impedance results with a single arc [22], as presented in the insets of Fig. 4c, d.
Note here that the overall resistivity was usually dominated by the grain-boundary resistivity for the PTCR ceramics so that the effect of semiconducting grains on the impedance results sometimes is not easily accessible from the -Z” vs Z’ plots [23]. The electric modulus, M *, is a persuasive demonstration to reveal the different electrical properties between the grain and grain boundary [22, 24]. Thus the data from impedance were reprocessed and presented in the electric modulus, M *, as following equation:
where ω is angular frequency 2πf and C0 = ε0A/l. ε0 is the permittivity of free space, 8.854 × 1014 Fcm−1. A is the area of the parallel plate and l is the thickness.
Different imaginary parts of the electric modulus, M”, were observed as shown in Fig. 4c, d. Only one peak can be observed in both -Z” and M” plots for the ceramic with x = 1.00 at ~103 Hz, which indicated that the bulk was electrically homogeneous and both the grains and grain boundaries were electrically insulated [22, 23]. On the other hand, both -Z” and M” plots for x = 1.03 showed a peak at a relatively low frequency of ~105 Hz, which was attributed to the high-resistance grain boundary. Besides, at higher frequency, an increasing incline in the M” plot suggested that a peak existed at a frequency above 30 MHz, which originated from the contribution of semiconducting grains [24], as depicted in Fig. 4d. Thus two peaks were expected in M” plots, which demonstrated that the ceramic with x = 1.03 was an electrically heterogeneous ceramic comprised by semiconducting grains and insulating grain boundaries [22, 25]. Namely, semiconducting grains were able to be formed at a lower sintering temperature and resistant to oxidation in the ceramics with x = 1.03.
3.3 Comparison study
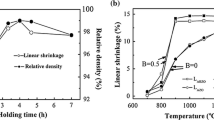
Note here that the particle size of the synthesized Bax-0.003La0.003TiO3 nanopowder was nearly the same (see Fig. 2), which indicated that the influence of the nano-size particle on the grain growth was approximate. Additionally, the Ba-solubility in BaTiO3 lattice is very limited (< 100 ppm) [19, 20], which was too low to result in the abnormal grain growth as x = 1.03. Consequently, the effect of BaCO3, second phase in the powder with x = 1.03, was taken into account. In the next experiment, an equivalent amount of BaCO3 (3 mol%), d10 = 0.48 μm, d50 = 1.36 μm, d90 = 3.39 μm, was added directly to the Bax-0.003La0.003TiO3 nanopowder with x = 1.00 to exclude the Ba-solubility but maintain the second phase BaCO3. The grains grew up again to about 1.2 and 2.1μm and the densification was enhanced as BaCO3 was added into the powder with x = 1.00, as shown in Fig. 5. Both the average grain size and relative density became close to the ceramics with x = 1.03.
Furthermore, compared with the ceramics with x = 1.00, the electrical properties were also changed by the addition of BaCO3, especially at low sintering temperature, as shown in Fig. 6. Complex impedance was conducted to analysis the effect of BaCO3 on the electrical property of grains and grain boundaries shown in Fig. 6a, b. It can be found that one arc was observed in the -Z” vs Z’ plot, but two peaks were also expected in the electric modulus, M”, plot for the ceramics sintered at 1075 °C and reoxidized at 800 °C, similar to the ceramic with x = 1.03 (see Fig. 4d). This result suggested that an electrically heterogeneous microstructure was able to be formed at a lower sintering temperature as BaCO3 was induced. Due to the formation of semiconducting grains, the insulating property disappeared, meanwhile, the RT resistivity decreased to about 5.3 × 103 Ωcm and PTCR effect was observed as presented in Fig. 6c, d. Based on the above narratives, it can be concluded that BaCO3 should be responsible for the abnormal grain growth and formation of semiconducting grains at a relative low sintering temperature.
3.4 Analysis of grain growth and electrical properties
TG-DTA was employed to analyze the thermal behavior for the powder with x = 1.00 and 1.03 under N2 atmosphere as shown in Fig. 7. Clearly, different weight loss and endothermic events occurred between the two powders. As x = 1.00, the smooth TG-DTA curves (Fig. 7a) indicated that the composition was relatively thermostable under N2 atmosphere. However, two distinct endothermic peaks (peak A and B) were observed in the DTA curve as x = 1.03. Meanwhile, a weight loss occurred together with the peak A at temperature above ~800 °C shown in Fig. 7b. To further clarify this endothermic event (peak A), green pellets were calcined at 700 and 900 °C in N2 atmosphere for 2 h, which were then pulverized into powder for phase identification. The peak of BaCO3 disappeared, meanwhile, the peak of Ba2TiO4 was observed as shown in Fig. 8. Combining the results of TG-DTA and XRD, it can speculate that the endothermic peak A corresponds to a chemical reaction, which was also reported pervious [26, 27]:
Note here that the BaCO3 has a low melting point of only 811 °C, but Valant et al. found that the grain growth could not be promoted by BaCO3 only. Because the melted BaCO3 reacted rapidly with the BaTiO3 matrix and then was consumed when the sintering temperature was elevated up to the melting point as depicted in Eq. 2 [26]. However, oxygen vacancies \(\left( {{{{\mathrm{V}}}}_{{{\mathrm{O}}}}^{ \bullet \bullet }} \right)\) can be easily created in BaTiO3 lattice during the reduction sintering through the equation below [14]
The lattice-diffusion coefficient is proportional to the concentration of \({{{\mathrm{V}}}}_{{{\mathrm{O}}}}^{ \bullet \bullet }\), as a result, the sintering was kinetically enhanced by the diffusion of \({{{\mathrm{V}}}}_{{{\mathrm{O}}}}^{ \bullet \bullet }\) [26]. A similar low-temperature sintering, named reactive liquid-phase sintering, has been achieved by many authors through the incorporation of Li+ into BaTiO3 lattice resulting in the formation of \({{{\mathrm{V}}}}_{{{\mathrm{O}}}}^{ \bullet \bullet }\) [27, 28]. Namely, the BaCO3 and the \({{{\mathrm{V}}}}_{{{\mathrm{O}}}}^{ \bullet \bullet }\) together triggered the reactive liquid-phase sintering leading to the abnormal grain growth in the Bax-0.003La0.003TiO3 ceramics with x = 1.03 at a relatively low temperature as shown in Fig. 3.
On the other hand, accompanying with the first peak, another endothermic peak B was presented in the DTA curve shown in Fig. 7b, which was not observed in the BaTiO3-BaCO3 system reported previously. According to the phase diagram for BaO-TiO2 system, the Ba2TiO4 phase was thermostable in BaTiO3 matrix indicating that no reaction occurred between BaTiO3 and Ba2TiO4 [19]. Considering that no weight loss was observed or too small to be detected in the TG curve in Fig. 7b, the second peak (peak B) was possibly induced by a variation of crystal structure. Combing with the above results that semiconducting grains could be formed after sintered at a relatively low temperature as x = 1.03 (see Fig. 6), it can speculate that an insulating phase \(( {{{{\mathrm{Ba}}}}_{1 - x}{{{\mathrm{La}}}}_x^ \bullet {{{\mathrm{Ti}}}}_{1 - x/4}^ \times {{{\mathrm{V}}}}_{{{{\mathrm{Ti}}}}\,x/4}^{\prime \prime \prime \prime }{{{\mathrm{O}}}}_3})\) might be transformed into a semiconducting phase \(\left( {{{{\mathrm{Ba}}}}_{1 - x}{{{\mathrm{La}}}}_x^ \bullet {{{\mathrm{Ti}}}}_{1 - x}^ \times {{{\mathrm{Ti}}}}_x^\prime {{{\mathrm{O}}}}_3} \right)\), which can be described by a following simplified equation [29]:
This result was well consistent with previous studies that semiconducting grains were usually formed along with the abnormal grain growth [11], which was also observed in the Bax-0.003La0.003TiO3 ceramics with x = 1.03 shown in Fig. 3. Noted here that a trace of oxygen could be released during this process, but the concentration was too low to be detected by TG analysis as reported previously [30].
4 Conclusion
Semiconducting La-doped barium titanate ceramics were fabricated by reduction-reoxidation method from nanopowders synthesized by sol-gel method. Excessive barium element in the nanopowder was mainly existed in the form of BaCO3, which was found to be able to introduce two endothermic peaks in the DTA curve conducted in N2 atmosphere. Combining with the results of thermogravimetric, X-ray diffraction, electron microscopy and electrical measurements, it can speculate that the reactive liquid-phase sintering was triggered in the Ba-excessive La-doped ceramics sintered in N2 atmosphere. Then abnormal grain growth occurred and the insulating grains transformed into semiconducting grains during this process.
References
Rowlands W, Vaidhyanathan B (2018) Additive manufacturing of barium titanate based ceramic heaters with positive temperature coefficient of resistance (PTCR). J Eur Ceram Soc 39:3475–3483
Leng S, Cheng H, Zhang R, Gao C, Li Z (2021) Electrical properties of La-Mn-codoped BaTiO3-(Bi0.5Na0.5)TiO3 lead-free PTCR ceramics. Ceram Int 47:30963–30968
Ianculescu AC, Vasilescu CA, Crisan M, Raileanu M, Vasile BS, Calugaru M, Crisan D, Dragan N, Curecheriu L, Mitoseriu L (2015) Formation mechanism and characteristics of lanthanum-doped BaTiO3 powders and ceramics prepared by the sol-gel process. Mater Charact 106:195–207
Zu H, Chen T, Gao C, Gao C, Fu Q, Zhou D, Hu Y, Zheng Z, Luo W (2017) Abnormal reoxidation effects in Ba-excess La-doped BaTiO3 ceramics prepared by the reduction-reoxidation method. J Am Ceram Soc 100:2958–2964
Yan L, Fu Q, Zhou D, Wang M, Zheng Z, Luo W, Wang G (2019) Enhanced electrical properties of BaTiO3-based thermosensitive ceramics for multilayer chip thermistors applications by addition of (Bi0.5Na0.5)TiO3. Ceram Int 45:19113–19119
Brutchey RL, Cheng G, Gu Q, Morse DE (2008) Positive Temperature Coefficient of Resistivity in Donor‐Doped BaTiO3 Ceramics derived from Nanocrystals synthesized at Low Temperature. Adv Mater 20:1029–1033
Zhao Q, Gong H, Wang X, Chen I-W, Li L (2016) Superior Reliability Via Two‐Step Sintering Barium Titanate Ceramics. J Am Ceram Soc 99:191–197
Gao C, Fu Q, Zhou D, Zu H, Chen T, Xue F, Hu Y, Zheng Z, Luo W (2017) Nanocrystalline semiconducting donor-doped BaTiO3 ceramics for laminated PTC thermistor. J Eur Ceram Soc 37:1523–1528
Luan W, Gao L, Kawaoka H, Sekino T, Niihara K (2004) Fabrication and characteristics of fine-grained BaTiO3 ceramics by spark plasma sintering. Ceram Int 30:405–410
Niimi H, Mihara K, Sakabe Y, Kuwabara M (2007) Influence of Ba/Ti ratio on the positive temperature coefficient of resistivity characteristics of Ca-doped semiconducting BaTiO3 fired in reducing atmosphere and reoxidized in air. J Am Ceram Soc 90:1817–1821
Drofenik M, Makovec D, Zajc I, Langhammer HT (2002) Anomalous grain growth in donor-doped barium titanate with excess barium oxide. J Am Ceram Soc 85:653–660
Levi RD, Tsur Y (2005) The effect of oxygen vacancies in the early stages of BaTiO3 nanopowder sintering. Adv Mater 17:1606–1608
Wang X, Chan HL-W, Choy C-L (2004) Positive temperature coefficient of resistivity effect in niobium-doped barium titanate ceramics obtained at low sintering temperature. J Eur Ceram Soc 24:1227–1231
Zu H, Fu Q, Gao C, Chen T, Zhou D, Hu Y, Zheng Z, Luo W (2018) Effects of BaCO3 addition on the microstructure and electrical properties of La-doped barium titanate ceramics prepared by reduction-reoxidation method. J Eur Ceram Soc 38:113–118
Niimi H, Mihara K, Sakabe Y, Kuwabara M (2007) Preparation of Multilayer Semiconducting BaTiO3 Ceramics Co-Fired with Ni Inner Electrodes. Jpn J Appl Phys 46:6715–6718
Niesz K, Ould-Ely T, Tsukamoto H, Morse DE (2011) Engineering grain size and electrical properties of donor-doped barium titanate ceramics. Ceram Int 37:303–311
Zhou D, Zhao D, Fu Q, Hu Y, Jian G, Cheng X, Shen X (2012) Particle sizes effects on electrical properties and densification of laminated Ba1.002La0.003TiO3 ceramics. Ceram Int 39(3):2457–2462
Aghayan M, Zak AK, Behdani M, Hashim AM (2014) Sol-gel combustion synthesis of Zr-doped BaTiO3 nanopowders and ceramics: Dielectric and ferroelectric studies. Ceram Int 40:16141–16146
Lee S, Randall CA, Liu ZK (2007) Modified phase diagram for the barium oxide–titanium dioxide system for the ferroelectric barium titanate. J Am Ceram Soc 90:2589–2594
Hu YH, Harmer MP, Smyth DM (1985) Solubility of BaO in BaTiO3. J Am Ceram Soc 68:372–376
Leng S, Li G, Zheng L, Cheng L, Zeng J (2011) Influences of Ba/Ti Ratios on the Positive Temperature Coefficient of Resistivity Effect of Y-Doped BaTiO3-(Bi1/2Na1/2)TiO3 Ceramics. J Am Ceram Soc 94:1340–1342
Irvine JTS, Sinclair DC, West AR (1990) Electroceramics: characterization by impedance spectroscopy. Adv mater 2:132–138
Beltrán H, Cordoncillo E, Escribano P, Sinclair DC, West AR (2004) Insulating Properties of Lanthanum-Doped BaTiO3 Ceramics Prepared by Low‐Temperature Synthesis. J Am Ceram Soc 87:2132–2134
Morrison FD, Sinclair DC, West AR (2001) Characterization of lanthanum-doped barium titanate ceramics using impedance spectroscopy. J Am Ceram Soc 84:531–538
Yoon SH, Kim H (2002) Space charge segregation during the cooling process and its effect on the grain boundary impedance in Nb-doped BaTiO3. J Eur Ceram Soc 22:689–696
Valant M, Suvorov D, Pullar RC, Sarma K, Alford NM (2006) A mechanism for low-temperature sintering. J Eur Ceram Soc 26:2777–2783
Valant M, Suvorov D (2004) Low-Temperature Sintering of (Ba0.6Sr0.4)TiO3. J Am Ceram Soc 87:1222–1226
Wang SF, Yang TC, Huebner W, Chu JP (2000) Liquid-phase sintering and chemical inhomogeneity in the BaTiO3-BaCO3-LiF system. J Mater Res 15:407–416
Makovec D, Drofenik M (2000) Microstructural changes during the reduction/reoxidation process in donor-doped BaTiO3 ceramics. J Am Ceram Soc 83:2593–2599
Drofenik M, Popović A, Irmančnik L, Kolar D, Kraševec V (1982) Release of oxygen during the sintering of doped BaTiO3 ceramics. J Am Ceram Soc 65:C-203-C-204
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No. 12004368), the China Postdoctoral Science Foundation (grant No. 2020M671859), the Anhui Provincial Natural Science Foundation (Grant No. 2108085QE198), the University Natural Science Research Project of Anhui Province (Grant No. KJ2019A0840, KJ2020A0652, gxyq2022071 and 2021kcszsfkc360) and the Talent Research Foundation of Hefei University (Grant No. 18-19RC36). The authors acknowledge the assistance by the Analytical and Testing Center of Huazhong University of Science and Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zu, H., Fu, Q., Cao, G. et al. Abnormal grain growth and electrical properties of Ba-excessive La-doped BaTiO3 ceramics prepared from nanopowder synthesized by sol-gel method. J Sol-Gel Sci Technol 106, 114–120 (2023). https://doi.org/10.1007/s10971-023-06052-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06052-7