Abstract
Ba0.8Sr0.2TiO3 (BST) nanopowder was successfully synthesized by Pechini sol-gel method under different temperatures. Not only were BaCO3 impurity phase production and transition temperature from cubic to tetragonal phase respectively set at 440 °C and 800 °C, but also barium (Ba) resided in a 12-coordinate site of crystal lattice was set at 580 °C by the techniques of X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR). In addition, gels at pH 3–8 were used to prepare BST ceramics and the ceramics morphology was characterized by scanning electron microscope (SEM). The interrelationship between morphology and electrical properties, which were associated with precipitation phase on the crystal boundary of BST ceramics, was studied in detail. Finally, the optimal electrical properties of the ceramics were comprehensively displayed in this work, i.e., εr = 8113.81, d33 = 78 pC/N, kp = 14.96%, and Qm = 85.08 at pH 5.

This research adopts Pechini sol-gel method for synthesis of Ba0.8Sr0.2TiO3 (BST) nanopowder and solid-state reaction for preparation of BST ceramics. Cubic to tetragonal phase evolution of nanopowder is detected when temperature above 800 °C. The optimal electrical properties of the ceramics are obtained with the as-synthesized nanopowder when gels at pH 5. The meaningful results exhibit for tunable microwave application.
Highlights
-
1.
Gelation and pyroreaction processes for Ba0.8Sr0.2TiO3 nanopowder were studied.
-
2.
Pyroreaction induced the nanopowder from cubic to tetragonal phase transition.
-
3.
Ceramics were prepared by Pechini sol-gel method with different pH values.
-
4.
Interrelationship of ceramics morphology and electrical properties was shown.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Barium titanate (BT) ceramic with perovskite structure had been extensively studied for the palmary electrical properties as well as ubiquity in piezoelectric materials [1]. Either isovalent or aliovalent modification in BT solid solution very often underlie the decrement on transition temperature and dielectric dissipation factors, and the increment on electrical properties [2]. It presented that barium strontium titanate solid solution had been diffusely applied into microwave devices including wireless communications (microwave phase shifter, delay lines dielectric filters, and voltage-controlled oscillators), varactors, multilayer ceramic capacitors, dynamic random access memories, etc [3,4,5]. Comparing with other divalent ions, strontium (Sr), which was doped in BT ceramic, had improved the piezoelectric coefficient (d33) realistically [6]. Further, the high permittivity, low dielectric loss, and high d33 are needed for these tunable microwave applications [7, 8]. Besides, barium strontium titanate ceramic is deemed to be a irreplaceable member among lead-free piezoelectric ceramic whose electrical properties were comparable to lead-based ceramics [7, 9]. However, the permittivity and piezoelectric coefficients of barium strontium titanate ceramic are inferior to lead-based ceramic for the moment, the Curie temperature (TC) of barium strontium titanate based ceramic is lower than lead-based ceramic, and the thermal dynamic stabilization is unsatisfactory [10]. In this study, we aimed on pinpointing the optimal condition to prepare barium strontium titanate ceramic of highly admirable electrical properties.
There are several techniques to prepare barium strontium titanate ceramic, such as hydrothermal, co-precipitation, spray pyrolysis, reverse micelles, combustion, and sol-gel methods [11,12,13]. Pechini sol-gel method is considered as a representative part of sol-gel method [14]. It means that the liquid precursor should be prepared by metal salt, ethylene glycol, as well as citric acid under low-temperature, transformed into a sol, and finally to a gelatin [15]. In this work, Ba0.8Sr0.2TiO3 (BST) nanopowder was synthesized by Pechini sol-gel method and ulteriorly sintered into ceramics under ordinary pressure. During sol formation process, the ionizing equilibrium of citric acid and the coordination equilibrium existed in solution compound of citric acid and metal ions. Act as a crucial raw material, citric acid’s ionization equilibrium is enormously influenced by the pH condition of the solution compounds (Eqs. 1–3) [16]. The gelation process directly impacts the crystal phase structure of BST nanopowder. Purity crystal phase structural nanopowder contributes to synthesize high tenability and high electrical property ceramics. Hence, pH conditions of the solution compounds and temperature condition for nanopowder were studied to obtain purity crystal phase structure BST nanopowder, improving the electrical properties of BST ceramic in this study.
2 Experimental
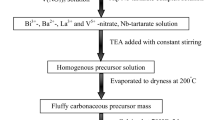
Ethylene glycol (Sinopharm Chemical Reagent Co., Ltd, 99.0% purity) and citric acid (Sinopharm Chemical Reagent Co., Ltd, 99.5% purity) were mixed by 4:1 ratio, accompanying with the magnetic stirring under the temperature of 55 °C. The Ti(OBu)4 (Sinopharm Chemical Reagent Co., Ltd, 98.0% purity) was added into the mixture as soon as the citric acid was completely dissolved to make homogeneous modified precursor. The polycondensation reaction was affirmed to end after 3 h stirring. Then Barium acetate (Sinopharm Chemical Reagent Co., Ltd, 99.0% purity) and strontium nitrate (Sinopharm Chemical Reagent Co., Ltd, 99.5% purity) were co-doped into the precursor successively. After stirring for 1 h, the solation process finished. Under the temperature of 110 °C, the sol was oven dried for 24 h. Furthermore, the structurally stable gel was formed after 12 h maturing under the room temperature. The weight loss and differential thermal analysis of gel (dried at 200 °C) were measured in a Thermogravimetric analysis/differential scanning calorimeter (TG/DSC, NETZSCH, STA 449 F5, DE) from room temperature to 1200 °C. The crucibles of gelatin were calcined into dark brown powder under the temperature from 350 °C to 470 °C in the furnace with the confirmed procedure. In addition, 440 °C was confirmed to be the calcination temperature for dark brown powder. Next, they were calcined into nanopowder under the temperature designated from 500 °C to 1000 °C. In order to illuminate the phase and component transitions of the powder, we adopted X-ray diffraction (XRD, Mini Flex 600), and Fourier transform infrared spectroscopy (FT-IR, Nicolet IS-50) for mutual corroboration.
After exploring the temperature for both reaction and grain growth process in the nanopowder, the impacts of pH conditions on structure and electrical properties were also investigated. The gels at pH 3–8 were characterized by a precise pH instrument (pHS-3C, INESA). These gels were processed as nanopowder under the same temperatures mentioned before. After that, the discs, 1 mm diameter and 1.5 mm thickness, were pressed under a 20 MPa pressure intensity using polyvinyl alcohol (PVA) of 5–6 wt.% as a binder. PVA was burned off at 650 °C for 2 h. Then the samples were sintered at 1260 °C for 4 h and the heating and cooling rates are respectively 3 and 4 °C/min. For the measurement of electrical properties, silver slurry firing on both sides of the sintered ceramics was under 570 °C for 12 min [17]. The permittivity (εr) and dielectric loss (tan δ) were both tested at room temperature under regularly different frequencies by a LCR analyzer (E4900A, Keysight). The εr and tan δ were tested at the frequency 10 kHz further by a precision impedance analyzer (Wayne Kerr) from 30 °C to 200 °C. BST ceramics were poled by a polarization installation (HYJH-4-5) under room temperature in a silicone oil bath for 40 min when dc field achieved to 3 kV/min. Twenty-four hours after poling, the piezoelectric constants of the ceramics were measured by piezo-d33 meter (ZJ-3AN). The d33, which were measured by the LCR analyzer (E4900A, Keysight), were used to calculate mechanic quality factor (Qm) and electromechanical coupling factor (kp) with the Onoe and Jumonji’s empirical Eqs. 4 and 5 [18]. Finally, the morphology on natural section of the ceramics was shown by scanning electron microscope (SEM, S-4800).
3 Results and discussion
3.1 Reaction process
3.1.1 Gelation process
As a precursor in hydrolysis, Ti(OBu)4 continuously reacts with H2O until Ti(OH)4 produced. In order to reduce rate of hydrolysis and obtain stable sol, the citric acid combined with the Ti(OBu)4 precursor as a chelating agent to form complexes with large steric hindrance [19]. For the sake of building a reticulate polymer, the dehydration polycondensation reaction and the dealcoholization polycondensation reaction are set off simultaneously. When the polycondensation reaction have accomplished, the metal ions are doped into the reticulate structure, which are combined with bridging oxygen bonds as Ti-O-Ti, Ba-O-Ba, and Sr-O-Sr. The water and organic solution still existed in the sol. During the aging process, the reticulate structure is starting to shrink, the condensation polymerization is keeping on, and the solution may lose at the same time [15]. That is the beginning of gelation procedure. According to the same mentality, the energy of citric acid in the combination with precursor is weakening as pH conditions increasing. Meanwhile, some of Ti(OBu)4 reprecipitates in the reticulate structure and the particles are contacted with each other to present one-dimensional linear structure, as shown in Fig. 1. It is useless to form stable solid solution and fine nanopowder.
Given this fact, some effects of different parameters, such as pH condition of gel and the calcination temperature, exist on morphology and particle size of products [20, 21]. As a consequence, the pH of gel strongly affects the agglomeration product and ulteriorly affects the electrical or optical properties, etc.
3.1.2 Pyroreaction process
The gelatin samples (dried under the temperature of 200 °C) were measured by TG/DSC to investigate the pyroreaction decomposition behaviors of the modified precursor in air and the results were shown in Fig. 2. From room temperature to 263.7 °C, a minor weight loss in TG curve accompanied with a weak endothermic peak in DSC curve in virtue of water evaporating decalescence [21]. It was clearly shown in the DSC curve that the first exothermic peak was detected at 323.6 °C with severe weight loss. Obviously, when the combustion stage was achieved, the polymer organics started to vaporize at 263.7 °C [22]. As the temperature elevated to 440 °C, the second endothermic peak had been discovered. In the mean time, the impurity phase was detected. The final exothermic peak at 580 °C showed that Ba-O and the impurity phase started to decompose. It was corroborated by the analysis of the FT-IR spectra and XRD patterns at a later stage [23]. Ultimately, Ba resided in a 12-coordinate site in the perovskite structure. In this conclusion, several temperatures that were adjacent to the temperatures mentioned before were selected to create powder and ceramics. The characterization of nanopowder that was calcined under the temperature from 55 °C to 1000 °C was accomplished.
The FT-IR spectra were shown in Fig. 3. The gels were created at 55 °C, dried at 200 °C, and respectively calcined into the dark brown powder from 200 °C to 470 °C. After that, the dark brown powders were calcined into nanopowders respectively from 500 °C to 1000 °C. The peaks ranged from 450 to 1000 cm−1 was identified to be three different kinds of vibration modes with TiO6 octahedral structures, which involved the Ti-OI stretching normal vibrations, Ti-OII ending normal vibrations, and TiO6 stretching vibration connected with barium [24]. Thus, the absorbance peaks of Ti-O at 503 cm−1 were detected and Ba-O (Fig. 3a) were detected around 423 and 856 cm−1 [25, 26]. The absorbance peaks were detected in frequency regime 1420–1600 cm−1 and the peaks at 1425 and 1565 cm−1 were identified to the asymmetrical and symmetrical vibration modes of (COO–) carboxyl group, respectively [24]. These groups might exist as ligands in the chelating agents due to the complexation reaction between citric acid and Ti(OBu)4 [27]. It was proved that the absorbance peaks in the range of 1000–1100 cm−1 might have the –OR groups in the combination between chelating agent and ions. The absorbance peaks of metal bridge oxygen bonds, such as Sr-O-Ti, Ba-O-Ti, and Ti-O-Ti, etc. range from 1300 to 1500 cm−1 [28]. It was evidently detected that the (–OH) groups had vaporized when the temperature achieved to 440 °C. The absorbance peaks of (COO–) carboxyl group, which were considered as an impurity phase (mentioned in Fig. 4), appeared in FT-IR peaks simultaneously (mentioned in Fig. 3b). From 55 °C to 700 °C, there was always keeping Ba-O in FT-IR graph at the wave number of 856–875 cm−1 [29]. In addition, the exothermic peak, detected at 580 °C (mentioned in Fig. 2), was authenticated to be the transformation for Ba-O from compound to crystal lattice (mentioned in Fig. 4). As the calcination temperature arising at 800 °C, the absorbance peaks of impurity phase and Ba-O were disappeared completely (mentioned in Fig. 3b). The results shown in the FT-IR graphs could be appropriately corresponded to the information in TG-DSC curves.
3.2 Structural studies
3.2.1 X-ray diffraction
The powder samples also characterized the phase transition under different calcination temperature conditions to confirm the phenomenon in FT-IR graphs and identify the optimal calcinations temperature ulteriorly. Figure 4 showed the XRD patterns of BST powder samples. In the Fig. 4a, all the major diffraction peaks were indexed according to the standard XRD patterns of the cubic BST phase (Pm-3m, cubic phase) and an impurity phase both transformed into the tetragonal BST phase (P4mm, tetragonal phase) by Jade 6.0 software. In Fig. 4b, the impurity phase, which was identified to be BaCO3 phase (Pnma, orthorhombic phase), was detected at 440 °C [30]. Meanwhile, BaCO3 phase (Pnma, orthorhombic phase) disappeared at 800 °C. In Fig. 4a, the types of phase included cubic BST and the BaCO3 impurity phase at 700 °C. There was no impurity phase observed and the principle crystal phase transformed from cubic to tetragonal phase as soon as the calcination temperature achieved 800 °C. The phenomenon was corresponding to the same things shown in FT-IR spectra. The BaCO3 impurity phase appeared at 440 °C and had an absorbance peak at the wave number of 1425 cm−1 in FT-IR spectra. It was disappeared at 800 °C when the absorbance peak of BaCO3 impurity phase disappeared at the same time. Furthermore, the absorbance of Sr-O-Ti vanished when the calcination temperature achieved 500 °C, but the diffraction peaks of BST phase still existed. It could be concluded that Sr2+ ions had dissolved in BaTiO3 matrix and formed a stable BST crystal phase structure. Thus, there was no doubt that the optimal temperatures for calcinations of dark brown powder and nanopowder were respectively set at 440 and 800 °C.
3.2.2 Scanning electron microscopy
The SEM images of the fractured surface morphology were shown in Fig. 5. The average grain size was estimated by linear intercept method. The average grain size was graduated as 1.17, 0.63, 0.50, and 1.25 μm, respectively, from Fig. 5a, d. Moreover, from Fig. 5a, c, it was observed that the grain size of the ceramics became smaller and more regular. The minimum grain size was detected at Fig. 5c. At pH 6–8, the grain size of nanopowder increased, the crystal boundaries of the ceramics grew blurred, and the section of ceramics even displayed intergranular fracture [31, 32]. These kinds of phenomenon in the ceramics were closely related to the structure of gels, which were prepared under different pH conditions. As mentioned previously, alkaline gels could engender the reprecipitation on the molecular reticulate structure and ulteriorly a linear aggregation. This kind of aggregation experienced the calcination and sintering procedure, and formed a precipitated phase on the crystal boundaries [33]. Furthermore, with increment of gel’s pH values, the situation of precipitated phase became more incurable and the intergranular fracture was detected in Fig. 5e, f.
3.3 Electrical properties
It was ulteriorly approved that the electrical properties and morphology of BST ceramics were influenced by crystal phase structure of BST nanopowder, which were prepared from gels at pH 3–8. The variation tendency of the εr and tan δ of BST ceramics are shown in Fig. 6 as the form of εr-frequency patterns from Fig. 6a, f. Both permittivity and dielectric loss of the BST ceramics were measured under incremental frequencies [34]. It showed that the decrement of εr and the fluctuation of tan δ were followed with the increment of frequency. The decrement of εr was attributed to the jumping frequency of electric charge carriers in the samples that could not follow the alternation of the applied AC electric field beyond a certain critical frequency [35]. This tendency of dielectric and relaxation polarization mechanisms was generally detected at high frequencies. The εr value of piezoelectric material depended on the mobility of domain walls and the population of domains [36]. Consequently, the increment of εr was due to the uniform grain size and the regular, as well as easy movement of domain walls [37]. For these reasons, εr of Fig. 6c at low frequencies was entirely higher than others and, by contrary, the dielectric loss was also relatively low in these patterns. In order to investigate the theoretical basis on the evolution of εr and tan δ, the temperature dependence of permittivity was shown in Fig. 7. The sharp permittivity peaked at pH 5, whose permittivity had achieved peak value (εr = 8113.81, Tc = 59 °C) accompanied with low dielectric loss and ferroelectric–paraelectric phase transformation temperature (Tc). Moreover, the permittivity peak of pH 3, 4, 6, 7, and 8 phase transition temperature range became broader, meaning the diffuse phase transition. As a typical characteristic of the relaxor ferroelectrics, the relaxor behavior due to the diffuse phase transition, which is engendered by polar-regions local spontaneous polarization [38, 39]. The phenomenon of polar-regions local spontaneous polarization at paraelectric phase was called polar nanoregions. Further, the disordered morphology and the expanded grain size of ceramics could produce polar nanoregions [39]. Consequently, the ceramics synthesized by the gel with different pH conditions ultimately led to the evolution of permittivity. In conclusion, the structural uniformity in ceramic at pH 5 really contributed to the dielectric property. These results essentially corresponded to the interrelationship of morphology and electrical properties as mentioned before.
The d33, kp, and Qm of BST ceramics were shown in Fig. 8. As the increment of pH values, the d33 values decreased first, then reached a peak value 78 pC/N, and eventually decreased again. BST ceramics were poled in silicon oil by dc field 3 kV for 40 min under the room temperature; thus, the d33 value was affected by poling levels first [37].
From the figure, the relatively smaller d33 of BST ceramic was obtained with the larger grain size, which was in line with the literature reported by Shao et al. [40]. The kp values almost decreased with the increment of the pH conditions. The optimal planar electromechanical property achieved at pH 5 with kp = 14.96% when pH conditions ranged from 4 to 8. However, the optimal kp values was obtained at pH 3 when pH conditions ranged from 3 to 8. It was because the highest density of these ceramics is speculated with the optimal kp values [40]. The Qm values increased sostenuto at pH 3–5 and decreased at pH 6–8. The growth in grain size engendered the intensive strain in domain movement, and ulteriorly in the increment of Qm values [41]. Thus, we could conclude that the optimal d33 value was 78 pC/N, Qm was 236.43, and the kp was 14.96% at pH 5.
4 Conclusion
BST nanopowders were calcined under different temperatures and the BST ceramics were synthesized with gels at pH 3–8. The BaCO3 impurity phase in BST nanopowder was detected under the temperature of 440 °C and disappeared under the temperature of 800 °C. The precipitation phase had an adverse effect on the electrical properties and morphologies, which were brought by the gels with different pH conditions. The optimal electrical properties of the BST ceramics were obtained, i.e., εr = 8113.81, d33 = 78 pC/N, kp = 14.96%, and Qm = 85.08 at pH 5. The interrelationship between the morphology and optimal electrical property of BST ceramics was also analyzed. The uniform population of domains and regular grain size as well as the distinct phase boundaries led to the increment in permittivity and d33. In addition, the excellent electrical properties of BST ceramics in this work could be used in the tunable microwave applications.
References
Mondal T, Majee BP, Das S, Sinha TP, Middya TR, Badapanda T, Sarum PM (2017) A comparative study on electrical conduction properties of Sr-substituted Ba(1−x)SrxZr0.1Ti0.9O3(x=0.00–0.15) ceramics. Ionics 23:2405–2416
Bian Y, Zhai J (2014) Low dielectric loss Ba0.6Sr0.4TiO3/MgTiO3 composite thin films prepared by a sol-gel process. J Phys Chem Solids 75:759–764
Stanculescu R, Ciomaga CE, Padurariu L, Galizia P, Horchidan N, Capiani C, Galassi C, Mitoseriu L (2015) Study of the role of porosity on the functional properties of (Ba,Sr)TiO3 ceramics. J Alloy Compd 643:79–87
Upadhyay RB, Annam S, Patel MR, Sharma AK, Mevada P, Joshi US (2016) Ba0.60Sr0.4TiO3 thick film derived by polymer modified MOSD route for tunable micro strip antenna applications. Ferroelectr Lett 43:25–33
Upadhyay RB, Annam S, Patel MR, Joshi US (2015) Influence of aliovalent doping on dielectric properties of Ba0.6Sr0.4TiO3 thin film for voltage tunable applications. Integr Ferroelectr 167:184–191
Li Y, Cheng HB, Xu HW, Zhang YX, Yan P, Huang T, Wang CM, Hu ZG, Ouyang J (2016) Electromechanical properties of (Ba,Sr)(Zr,Ti)O3 ceramics. Ceram Int 42:10191–10196
Pal J, Kumar S, Singh L, Singh M, Singh A (2017) Detailed investigation on structural, dielectric, magnetic and magnetodielectric properties of BiFeO3-BaSrTiO3 solid solutions. J Magn Magn Mater 441:339–347
Hossain SKS, Roy PK (2017) Structural and electro-magnetic properties of low temperature co-fired BaSrTiO3 and NiCuZn ferrite composites for EMI filter applications. J Mater Sci Mater El 28:18136–18144
Li P, Zhai JW, Shen B, Zhang SJ, Li XL, Zhu FY, Zhang XM (2018) Ultrahigh piezoelectric properties in textured (K,Na)NbO3-Based lead-free ceramics. Adv Mater 30:1705171
Tian YS, Gong YS, Meng DW, Deng H, Kuang BY (2015) Low-temperature sintering and electric properties of BCT-BZT and BCZT lead-free ceramics. J Mater Sci Mater El 26:3750–3756
Dong SP, Rao AVP, Komarneni S (1997) Titanate and barium/strontium titanate thin films from hydroxide precursors: preparation and ferroelectric behavior. J Sol Gel Sci Techn 10:213–220
Huang YH, Wu YJ, Li J, Liu B, Chen XM (2017) Enhanced energy storage properties of barium strontium titanate ceramics prepared by sol-gel method and spark plasma sintering. J Alloy Compd 701:439–446
Gharibshahian M, Nourbakhsh MS, Mirzaee O (2018) Evaluation of the superparamagnetic and biological properties of microwave assisted synthesized Zn & Cd doped CoFe2O4 nanoparticles via Pechini sol-gel method. J Sol Gel Sci Techn 85:684–692
Salavati-Niasari M, Soofivand F, Sobhani-Nasab A, Shakouri-Arani M, Hamadanian M, Bagheri S (2017) Facile synthesis and characterization of CdTiO3 nanoparticles by Pechini sol-gel method. J Mater Sci Mater El 28:684–692
Danks AE, Hall SR, Schnepp Z (2016) The evolution of ‘sol-gel’ chemistry as a technique for materials synthesis. Mater Horiz 3:91–112
Suárez-Toriello VA, Santolalla-Vargas CE, Reyes JADL, Vázquez-Zavala A, Vrinat M, Geantet C (2015) Influence of the solution pH in impregnation with citric acid and activity of Ni/W/Al2O3 catalysts. J Mol Catal A Chem 404–405:36–46
Zheng T, Ding Y, Wu JG (2106) Bi nonstoichiometry and composition engineering in (1−x)Bi1+yFeO(3+3y)/2-xBaTiO3 ceramics. RSC Adv 6:90831–90839
Onoe M, Jumonji H (1966) Useful formulas for piezoelectric ceramic resonators and their application to measurement of parameters. J Acoust Soc Am 41:1223–1223
Danish M, Ambreen S, Chauhan A, Pandey A (2015) Optimization and comparative evaluation of optical and photo catalytic properties of TiO2 thin films prepared via sol-gel method. J Saudi Chem Soc 19:557–562
Wu YT, Wang XF, Yu CL, Li EY (2012) Preparation and characterization of barium titanate (BaTiO3) nano-powders by Pechini sol-gel method. Adv Manuf Process 27:1329–1333
Hsieh TH, Yen SC, Ray DT (2012) A study on the synthesis of (Ba,Ca)(Ti,Zr)O3 nano powders using Pechini polymeric precursor method. Ceram Int 38:755–759
Maya JC, Chejne F, Bhatia SK (2016) Novel model for the sintering of ceramics with bimodal pore size distributions: application to the sintering of lime. AIChE J 63:893–902
Hasbullah NN, Lee OJ, Chyi JLY, Chen SK, Talib ZA (2017) Synthesis of BaTiO3 nanoparticles via hydrothermal method. Solid State Phenom 268:172–176
Jayalath S, Wu H, Larsen SC, Grassian VH (2018) Surface adsorption of suwannee river humic acid on TiO2 nanoparticles: a study of pH and particle size. Langmuir 34:3136–3145
Peperstraete Y, Amzallag E, Tetot R, Pascale R (2018) Ab initio study for the IR spectroscopy of PbTiO3 and PbZrO3, primary blocks of PbZr1-xTixO3. J Phys-Condens Mat 30:1–11
Hao YN, Wang XH, Kim JY, Li LT (2014) Rapid formation of nanocrystalline BaTiO3 and its highly stable sol. J Am Ceram Soc 97:3434–3441
Naeem A, Mahmood A, Iqbal Y, Ullah A, Mahmood T, Humayun H (2015) Dielectric and impedance spectroscopic studies on (Ba0.5Sr0.5)Mnx(Ti0.95Fe0.05)1−xO3 ceramics synthesized by using sol-gel method. J Alloy Compd 645:290–296
Attar AS, Sichani ES, Sharafi S (2016) Structural and dielectric properties of Bi-doped barium strontium titanate nanopowders synthesized by sol-gel method. J Mater Res Technol 6:108–115
Lopes CDCA, Limirio PHJO, Novais VR, Dechichi P (2018) Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl Spectrosc Rev 53:1–23
Liu SZ, Wang TX, Yang LY (2011) Low temperature preparation of nanocrystalline SrTiO3 and BaTiO3 from alkaline earth nitrates and TiO2 nanocrystals. Powder Technol 212:378–381
Raj R, Ashby MF (1975) Intergranular fracture at elevated temperature. Acta Metall Sin 23:653–666
Ortiz M, Suresh S (1993) Statistical properties of residual stresses and intergranular fracture in ceramic materials. J Appl Mech 60:77–84
Han XH, Li Y, Zhao YQ, Chi X, Zhang C, Bian LP, Sun JB, Zhang Y (2017) Microstructure and phase transition of Fe-24Cr-12Co-1.5Si ribbons. J Alloy Compd 731:10–17
Huang YQ, Du HW, Feng W, Qin HN, Hu QB (2014) Influence of SrZrO3 addition on structural and electrical properties of (K0.45Na0.51Li0.04)(Nb0.90Ta0.04Sb0.06)O3 lead-free piezoelectric ceramics. J Alloy Compd 590:435–439
Srikanth KS, Singh VP, Vaish R (2017) Pyroelectric performance of porous Ba0.85Sr0.15TiO3 ceramics. Int J Appl Ceram Tec 15:140–147
Shaw TM, Trolier-Mckinstry S, Mcintyre PC (1983) The properties of ferroelectric films at small dimensions. Annu Rev Mater Res 30:263–298
Mudinepalli VR, Feng L, Lin WC, Murty BS (2015) Effect of grain size on dielectric and ferroelectric properties of nanostructured Ba0.8Sr0.2TiO3 ceramics. J Adv Ceram 4:46–53
Gao WW, Lv J, Lou XJ (2018) Large electric-field-induced strain and enhanced piezoelectric constant in CuO modified BiFeO3-BaTiO3 ceramics. J Am Ceram Soc 101:2653–2665
Cheng S, Zhang BP, Zhao L, Wang KK (2018) Enhanced insulating and piezoelectric properties of 0.7BiFeO3-0.3BaTiO3 lead-free ceramics by optimizing calcination temperature: analysis of Bi3+ volatilization and phase structures. J Mater Chem C 6:3982–3989
Shao SF, Zhang JL, Zhang Z, Zheng P, Zhao ML, Li JC, Wang CL (2009) High piezoelectric properties and domain configuration in BaTiO3 ceramics obtained through the solid-state reaction route. J Phys D Appl Phys 42:9801–189801
Liu YW, Pu YP (2016) Dielectric properties, relaxor behavior and temperature stability of (1−x)(K0.4425Na0.52Li0.0375)(Nb0.87Ta0.06Sb0.07)O3−xBa0.4Sr0.6TiO3 ceramics. J Alloy Compd 693:118–125
Acknowledgements
We thank the Henan Provincial Department of Science and Technology Research Project (162102310122), the Key Project of Henan Province Colleges and Universities (19A430021), the Scientific Research Foundation of Graduate School of Xinyang Normal University (2018KYJJ17), and Nanhu Scholars Program for Young Scholars of XYNU, for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, Y., Cao, L., Chen, Z. et al. Impact mechanism of gel’s alkali circumstance on the morphologies and electrical properties of Ba0.80Sr0.20TiO3 ceramics. J Sol-Gel Sci Technol 90, 621–630 (2019). https://doi.org/10.1007/s10971-019-04959-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-04959-8