Abstract
Water treatment is a worldwide problem that can be solved by the interaction of light with nanoparticles like WO3 nanoparticles. However, most nanoparticles have low photocatalytic efficiency in acidic conditions like acidic effluents of factories. Therefore, in the study, WO3 nanoparticles are synthesized with the sol–gel route as a simple and low-cost method, and then the nanostructures are calcined at a different temperature to find an effective photocatalytic agent for acidic water treatment. For the purpose, optical and structural properties, crystallinity, thermodynamic stability, and hydrodynamic diameter of the nanostructures are investigated in the paper. Raman and FTIR spectra of all four samples have approximately similar peaks and indicate the formation of WO3. XRD and thermal analysis point to a transition in the crystal phase with a change in calcination temperature. According to FESEM, DLS, and optical analyses, the as-synthesized and calcined WO3 nanoparticles @300 °C have the smallest particle size, the most stability in a liquid medium, and the best optical properties among other samples. The photocatalytic activities of these samples are evaluated via degradation of methylene blue, MB, (10 ppm) using irradiated WO3 NPs (20 ppm) under diode laser light with a central wavelength of 405 nm and the power of 100 mW. The high photocatalytic efficiency is obtained using the as-synthesized sample in both neutral (57.3%,) and acidic (74.7%) conditions and unlike most nanoparticles, the photocatalytic efficiency of the sample in acidic conditions is noteworthy, which makes it a suitable candidate for the treatment of acidic effluents in factories.

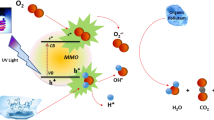
Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tungsten oxide is an n-type semiconductor with an indirect bandgap and it has some unique properties, which make it suitable for applications based on light absorption. Therefore, extensive research on this metal oxide is underway to find the optimized WO3 structure for photochromic application, cancer treatment, and drug delivery. Cancer is a worldwide disease with an increasing trend the world [1] and nanoparticles can be applied as sensitizing agents in non-invasive cancer treatment like photothermal therapy (PTT), photodynamic therapy (PDT), sonodynamic therapy (SDT), and targeted drug delivery systems (TDDS) [2]. Wastewater treatment is also a problem in today’s world community. Both global problems can be solved by the interaction of light with nanoparticles.
Tungsten oxide (WO3) has received special attention as one of the most promising agents of photodynamic therapy and semiconductor photocatalysts for the treatment of cancer and degradation of organic compounds due to its band structure. Researchers have shown that tungsten oxide nanoparticles have significant biophotocatalytic properties while being non-hazardous to the body [3]. Therefore, they can be exploited for their potential applications in nanobiotechnology. The mechanism of dyes photocatalytic degradation and cancer treatment using the light interaction with nanoparticles are similar, which can be briefly explained as follows: First, as shown in Fig. 1, the interaction of light with tungsten oxide nanoparticles leads to the transfer of electrons from the valance band to the conduction band, so that electron-hole pairs are produced. Electrons and holes interact with oxygen and water molecules to produce superoxide radical anion (•O2−) and hydroxyl radical (•OH), respectively. The reactive oxygen species (ROS) are highly reactive chemicals, which interact with dye molecules or the plasma membrane of cancerous cells and cause oxidative damage. The process can be formulated as follows [4]:
Due to the distinctive properties of tungsten oxide nanoparticles, extensive research has been done on the synthesis of the WO3 nanoparticles, composite, and doped WO3 nanostructures by different methods and the effects of synthesis parameters on the photocatalytic and photodynamic properties of these nanoparticles have been investigated [4]. In 2019, highly crystalline orthorhombic WO3 nanoparticles have been synthesized using an ultrasonic-assisted technique and then dextran as an additional steric stabilizer has been applied to enhance the stability of WO3 nanoparticles in biological media and to reduce theirs in Vivo toxicity [5]. The cytotoxicity of pure WO3 (without stabilizer) and dextran-stabilized WO3 has been studied in dental pulp stem (DPS) cell lines and breast cancer (MCF-7) cell lines. The results declare that both synthesized nanoparticles have low cytotoxicity and genotoxicity for both cells. Also, both WO3 nanoparticles were relatively safe up to the WO3 concentration of 1000 μg mL−1. In 2021, in Vivo treatment of relatively radioresistant breast cancer has been reported by applying near-infrared light irradiation at 1064 nm to tungsten oxide and a rather effective therapeutic outcome has been achieved [6]. In another work in 2021, WO3 nanocubes, nanobars, and nanobricks have been synthesized by hydrothermal method and then antimicrobial and anti-cancer activities of WO3 nanoparticles have been investigated against different micro-organisms and KB cell lines, respectively [7]. The results of the study confirm the effectiveness of anti-cancer and antimicrobial activity of WO3 nanostructures. Also, the WO3 nanostructures are safe for human life.
In 2016, tungsten oxide nanoparticles have been synthesized using the precipitation method and the nanoparticles have been calcined at 500 and 600 °C [8]. After that, the photocatalytic activities of the samples have been studied using the degradation of dye solutions (methylene blue, MB) under visible light irradiation. The results reveal that the photocatalytic efficiency of the WO3 nanoparticles calcinated at 500 °C is higher than 600 °C. In another work, WO3 hollow microspheres have been synthesized by immersing SrWO4 microspheres in a concentrated HNO3 solution, followed by calcination at 300, 400, 500, 600, and 700 °C [9]. Then, the photocatalytic activities of the samples have been investigated using the degradation of neutral dye solutions (rhodamine B, RhB) under visible light irradiation. The results declare that the highest photocatalytic efficiency of WO3 hollow microspheres is related to the sample calcinated at 300 °C followed by a non-calcined sample. The results of this study also show that the specific surface area (SSA) of the nanostructures decreases with increasing calcination temperature and this phenomenon can explain the decrease in photocatalytic activity by increasing the calcination temperature. In 2021, the effect of monochrome light (at 470, 520, and 620 nm) on the photocatalytic activity of commercial tungsten oxide nanopowder has been investigated and the results demonstrated that interaction of the radiation at 470 nm with WO3 nanopowder leads to the highest MB degradation or the photocatalytic activity of the nanopowder [10]. In 2016, pure and silver doped tungsten oxide nanoparticles have been produced by microwave irradiation method and the photocatalytic activities of the samples have been studied using degradation of MB solutions under visible light irradiation [11]. Their results show that silver doping causes a considerable red shift in the absorption edge, a decrease in the energy bandgap, and an increase in the photocatalytic activity of WO3 nanoparticles. However, silver doping is not economically justified due to the high cost of Ag precursors. In 2020, spherical and rod-like tungsten oxide embedded in Sodium Alginate/Polyvinylpyrrolidone (SA/PVP/WO3) nanocomposite has been produced and the photocatalytic activities of the samples have been studied via degradation of MB solutions under LED visible light irradiation [4]. Their results revealed that the photocatalytic efficiency of the nanocomposite containing rod-like WO3 is better than spherical WO3. In 2019, indigo dye/graphene/WO3 nanocomposite has been synthesized by hydrothermal method and the photocatalytic activity of the sample has been studied using the degradation of MB solutions (with pH = 3, 7, and 11) under sunlight irradiation [12]. The highest photocatalytic efficiency of the WO3 nanocomposite was achieved at the solution pH of 11 followed by the neutral solution pH. However, the photocatalytic efficiency of the nanocomposite was significantly low at the acidic solution pH (less than 5% after exposing sunlight to 30 mg of the dispersed nanocomposite at 100 ml MB with a concentration of 10 ppm). Therefore, the nanocomposite had not enough photocatalytic activity in acidic environments such as some factory effluent.
Although various synthesis methods such as hydrothermal, sol–gel, vapor deposition, pulsed laser deposition, acidification, electrochemical, and sonochemical methods have been used for producing WO3 nanoparticles [13,14,15]. The sol–gel method has some advantages such as the ability to produce high-quality and uniformly distributed nanoparticles at low cost and without requiring expensive and advanced types of equipment [16]. Therefore, sol–gel is selected in this study as a suitable method for the synthesis of WO3 nanostructures.
As mentioned, the photocatalytic activity of most nanoparticles is significant under alkaline and neutral conditions, while their photocatalytic activities in acidic media are negligible. Wastewater from industrial processes is acidic in many cases and nanoparticles with high photocatalytic activity are required to treat these waters. To the best of our knowledge, the use of metal oxides such as tungsten oxide to dye photodegradation of acidic wastewater is not effective and low photocatalytic efficiency is obtain for acidic water treatment.
So, this study is devoted to produce a safe and efficient photocatalytic agent based on WO3 nanoparticles for treatment of acidic wastewater like factory effluent. For the purpose, WO3 nanoparticles were synthesized with sol–gel method as a simple and low-cost method and calcined at different temperature. Then, the structural, optical, and photocatalytic activities of the synthesized nanoparticles were investigated to find the appropriate photocatalytic agent for treatment of the acidic water. ROS productions are examined in both acidic (pH = 5.4) and neutral (pH = 7.4) mediums via degradation of MB solutions under diode laser irradiation at 420 nm to find the high photocatalytic efficiency in acidic solutions. The results confirm that one of the WO3 nanoparticles synthesized using the sol–gel method has high MB photodegradation in acidic solution through the laser light interaction with the nanoparticles and can be a good candidate for treatment of acidic wastewater.
2 Experimental methods
2.1 Synthesis of WO3
WO3 nanostructures were synthesized using the sol–gel method. 3 gr sodium tungstate dehydrate (Na2WO4.2H2O) was dissolved in 100 ml deionized water. HCl (2 M) was added dropwise to the solution to adjust the pH at 1. The solution was stirred for 15 h at a temperature of 50 °C to obtain the gel. The gel was then kept in an oven at the temperature of 80 °C for 24 h, then the precipitate was washed three times with deionized water and finally ethanol to remove impurities. It was then dried in an oven at 80 °C for 24 h. The growth mechanism of WO3 nanostrusture at similar synthesis conditions has been reported and the relating chemical reactions have been suggested [17]. To investigate the effect of calcination temperature, the resulting yellow powder was calcined for 90 min at different temperatures of 300, 500, and 700 °C. All calcined WO3 structures were green powders and were labeled as 300 T, 500 T, and 700 T according to calcination temperature. The as-synthesized sample was labeled as NC.
2.2 Characterization of the samples
To determine the crystal structure of the samples, X-ray diffraction (XRD) analysis was done using the D8 Advanced Bruker system with Cu-Kα radiation (λ = 0.15406 nm). To investigate the type and intensity of chemical bonds, Fourier transform infrared spectroscopy (FTIR) was employed using the Shimadzu model 8400S and Transmission spectra of the samples were recorded in the wavenumber range of 400–4000 cm−1. The morphology and the particle size of the samples were determined using field emission scanning electron microscopy (FESEM) with the TESCAN model MIRA3 Microscope. The optical spectra of the samples were measured using both UV-Vis-NIR spectroscopy (Shimadzu 1800 UV-visible165-PC double-beam spectrophotometer) and diffuse reflection spectroscopy (Avaspec-2048-TEC). The hydrodynamic diameter of WO3 nanostructures was measured using dynamic light scattering (DLS) with the Avaspec-2048-TEC device. Thermal gravimetric analysis (TGA) was performed up to 1000 °C using LINSEIS 70/2171 device.
2.3 Photocatalytic degradation of Methylene blue
Methylene blue (MB) dye was used as an organic pollutant to study the photocatalytic and photodynamic activity of the synthesized WO3 nanoparticles. For this purpose, a solution of 10 ppm MB was made with the dissolution of 0.001 g MB in 100 cc deionized water. The MB degradation was evaluated under 405 nm laser irradiation in the presence of tungsten oxide nanoparticles for both NC and 300 T samples. The photocatalytic performance of these samples was evaluated under two different pH conditions; acidic (pH = 5.4; conditions of cancer cell regions) and neutral (pH = 7.4; normal body cell conditions) to find the structure with the highest ROS productions under these conditions. For this purpose, 0.001 g of NC and 300 T nanoparticles were added to 50 cc of 10 ppm MB solution and placed on a magnetic stirrer in the dark for 10 min, then irradiated with a 405 nm laser. After 10 min irradiation, the sample was centrifuged at 4000 rpm for 7 min. The resulting solution was vacuum poured into an Erlenmeyer flask to separate the possible remaining nanoparticles from the MB solution. Finally, the absorption spectra of the MB solution were taken using a UV-Visible spectrophotometer. This process was repeated every 10 min for 1 h and every 20 min for the second hour.
The degradation rate of methylene blue is a measure of ROS production due to the interaction of lasers and nanoparticles. The rate of methylene blue degradation can be calculated as follows:
where A0 is the initial absorption value and At is the absorption value at time t.
3 Results and discussion
3.1 Crystallinity
Figure 2 shows XRD patterns of the non-calcined and calcined WO3 nanoparticles at different temperatures of 300, 500, and 700 °C. The existence of diffraction peaks verifies the formation of crystalline structure in the samples. From the results, it is revealed that the structure of the non-calcined sample consists of two crystalline phases, orthorhombic WO3.H2O and hexagonal WO3.0.33H2O, as determined from X’pert highscore software. The orthorhombic WO3.H2O phase is grown preferentially in the direction of (111) which appears as an intence peak at 2θ = 25.6° in the XRD pattern of sample NC. It should be mentioned that presence of hexagonal WO3.0.33H2O phase is less dominant and only low intensity peaks are detected relating to this crystalline phase. The peak at 2θ = 27.2° corresponds to (121) reflection plane of this phase. The percentage of different crystal phases are determined quantitatively using MOUD software by simulating the XRD pattern. The phase percentage of orthorhombic WO3.H2O and hexagonal WO3.0.33H2O are obtained % 92.19 and %7.80, recpectively. For the sample 300 T, a phase transition occurs upon the calcination process so that both monoclinic WO3 and hexagonal WO3.0.33H2O phases are formed in this structure. However, the intensity of diffraction peaks for this sample is lower than others, which suggests lower degree of crystallinity in this sample. The phase transition might be a probable reason for the lower crystalline quality of the sample 300 T since the more stable monoclinic phase has not been completely formed at this condition yet. Here, the phase percentage of monoclinic WO3 and hexagonal WO3.0.33H2O phases are %24.20 and %75.79, recpectively. This means that the hexagonal phase is dominat phase in this sample. In the XRD pattern of sample 500 T, sharp and intence peaks verifies formation of high-quality crystalline structure that is well matched with monoclinic WO3 and preferential orientation of (002) appeared at 2θ = 23.1°. Further increase of sample temperature from 500 °C to 700 °C just leads to more crystal growth which reflets in significant enhouncement of peak intensities with no more phase change.
It is known that monoclinic structure is the most stable crystalline phase of WO3 at high temperatures [18], so that metastable phases are expected to transform into monoclinic phase upon calcination process. This phenomenon occurs due to minimization of the surface Gibbs energy/entropy of the structure and thus reaching more stability. From the XRD results, the phase transformations are verified when the orthorhombic phase changes to monoclinic with calcination at 300 °C and monoclinic/hexagonal changes to monoclinic with increment of calcination temperature from 300 °C to 500 °C. The change of crystalline phase due to calcination has been also reported in WO3 NPs synthesized using the hydrothermal and sol–gel methods [17,18,19]. Moreover, heating process is expected to evaporate the hydrate groups in the structure. This effect is also observed from the XRD pattern of samples by phase transitions from hydrated structures (orthorhombic WO3.H2O, hexagonal WO3.0.33H2O) to unhydrated structure (monoclinic WO3) upon increasing calcination temperature.
The XRD patterns of all samples are simulated using MOUD software and the crystalline properties of the sample such as the percentage of different crystal phases and lattice constants are derived as presented in Table 1. Moreover, the mean crystallite size of each sample are calculated using Deby-Scherrer formula [20] and gathered in Table 1. The mean crystallite size of different phases in terms of calcination temperature is shown in Fig. 3. It is seen that the crytallite size of hexagonal/monoclinic phase increased with increasing calcination temperature. At calcination temperatures above 300 °C single phase material is formed and grown.
3.2 Morphology
Figure 4 shows FESEM images and particle-size distribution histogram of non-calcined and calcined WO3 samples at different temperatures. The image of the NC sample indicates the formation of agglomerated irregular shape particles with an average size of ~ 600 nm. After calcination of the sample at 300 °C, the average particles reduces to ~530 nm and a narrower distribution of particle size is obtained. In the image of NC and 300 T samples, agglomerates of small particles are observed in which the size of agglomerates are relatively larger for NC sample. Increasing the calcination temperature from 300 to 500 °C leads to a reduction of particle size to 511 nm. These changes might be due to the evaporation of structural water and the formation of a new crystal phase as verified by XRD results. However, a further increase in calcination temperature up to 700 °C increases the particle size to ~750 nm, which can be related to further growth of the monoclinic phase as also shown in the XRD results. It was noticed that trace of small particles, observed in the non-calcined and 300 T samples, are hardly detected for the samples 500 T and 700 T. This might be due to particle growth, Ostwald ripening or grain sintering occurred at elevated temperatures. These processes usually occuer to decrease the surface to volume ratio and therefore suface energy of the structure. The effect of calcination temperature on the morphology of WO3 nanoparticles has been studied by Tijani et al. and their results are consistent to what (a) we observed here [21].
3.3 Chemical structure and vibration modes
Figure 5a shows FTIR spectra of non-calcined and calcined samples at different temperatures. A wide and intense absorption band in the range of ~470–1000 cm−1, which is characteristic of WO3 structure [22], and a peek at ~401 cm−1 related to the wagging vibration mode of W–O [23] are observed for all samples. This suggests that all samples have a similar chemical structure. The band in the range of 470–1000 cm−1 is usually regarded as a combination of absorption peaks related to stretching vibrations of W = O, W–O, W–O–W, O–W–O, and W–O–O [22, 23]. For more detailed analysis, this band is deconvoluted to its component peaks by using Origin Pro software. A typical deconvolution of this band is shown in Fig. 5b for the sample 500 T. It is seen that this wide band consists of absorption peaks at 624, 646, 889, and 1115 cm−1. The first two peaks are corresponding to the stretching vibration of W–O–W bond. The peak at 889 cm−1 is related to O–W–O stretching mode and the small peak at 1115 cm−1 is related to the stretching vibrations of W–O and W=O [23, 24]. Also, another two absorption bands at 1530–1650 cm−1 and 3310–3450 cm−1 regions are present in the spectra of NC and 300 T samples which are very weak in the spectra of the samples calcined at higher temperatures. These absorption peaks are associated with wagging and stretching vibrations of H–O–H bond [25], respectively. It was expected that the intensity of these peaks greatly decreases at high calcination temperatures due to the evaporation of structural water. Therefore the FTIR results are consistence with XRD analysis that verifies the phase change from hydrated orthorhombic/hexagonal WO3 to unhydrated monoclinic WO3 with increasing the calcination temperature.
To further investigate the chemical bond structure of the samples, Raman scattering spectroscopy was carried out and the Raman spectra of the samples are shown in Fig. 6. The main features of the Raman spectrum are similar for all samples except for some variations in peaks intensities and widths. This implies that the chemical structure of the samples is not changed significantly under the calcination process. The typical Raman spectrum of the samples consists of five peaks positioned at around 274, 327, 708, 800, and 948 cm−1 corresponding to υ(O–W–O), δ(W–O–W), γ(O–W–O), υ(W–O–W), and υ(W=O), respectively [17, 26, 27]. It is noticeable that the intensity of Raman peaks is greatly enhanced and their width is decreased as calcination temperature is increased, which is indicative of crystalline quality improvement of the samples. This result is consistence with increment of peak intensities with increasing calcination temperature from 300 to 700 °C observed in XRD results. It is seen that no extra peaks are detected in the Raman spectra due to calcination. It should be mentioned that small shifts in Raman peak positions are observed as a result of some changes in bond lengths and/or lattice strain.
3.4 Optical characteristics
UV-Vis-NIR and DRS spectroscopies are known as useful techniques for optical characterization of materials and determination of their optical constants such as energy gap. The absorption peak width in the UV-Vis-NIR spectrum provides some information about the particle size distribution of the sample such that a narrower absorption peak is related to a more uniform distribution of particle size. For experimental measurement, 0.0075 gr of the sample was dispersed in 60 ml DI water under ultrasound irradiation (100 Hz) for 3 min. Then, UV-Vis-NIR spectra of the dispersed samples were recorded in the range of 200–900 nm as shown in Fig. 7a. It is observed that increasing calcination temperature leads to a decrease in the absorption intensity of the samples. Therefore, the NC and 700 T samples have the most and least absorption in the whole range, respectively. Moreover, the absorption peak experiences a red-shifted with increasing the calcination temperature that might be caused by variation of particle size during the calcination process [28]. Furthermore, it was noticed that the FWHM of absorption peak increased upon calcination from 300 °C to 700 °C. This suggests that the distribution of dynamic diameter of WO3 nanoparticles in the aqueous solution becomes wider with increasing the calcination temperature.
DRS measurement was also done on the samples to further study their optical properties. This method is usually employed for powder samples where the light diffuse reflectance occurs. The diffuse reflectance spectra of the samples are shown in Fig. 7b. As you can observe, the reflectance in the UV region is low for all structures. Also, the calcination process leads to the enhancement of reflectance in this region. Indeed, the reflectance is around 0.9 in the visible region for all samples, although the 300 T sample shows lower absorption in the Vis-NIR region and reflectance reaches R = 0.75 at 808 nm.
Kubelka-Munk function, \(F\left( R \right) \,=\, \left( {1 \,-\, R^2} \right)/\left( {2R} \right)\), is proportional to the absorption coefficient, α, and is commonly used in Tauc method. To determine the energy gap of the samples according to Tauc method [29, 30], the plots of (αE)1/2 versus energy for non-calcined and calcined WO3 nanoparticles are shown in Fig. 8. The obtained values of energy gap are 2.74, 2.9, 2.75, and 2.5 eV for the samples NC, 300 T, 500 T, and 700 T, respectively. The calcination process at 300 °C causes an increment in Eg, however, increasing calcination temperature from 300 to 700 °C leads to a noticeable decrease in the energy gap [31].
UV-Vis-NIR spectroscopy shows the highest absorption of UV light for the NC sample. Practically, we concern with dispersed nanoparticles in the solution for applications such as removal of contaminants from factory wastewater by photocatalysts. Therefore, in this case, UV-Vis-NIR results are more reliable than DRS. Thus, the non-calcined sample can be a suitable candidate for the mentioned application.
3.5 Thermal properties
Thermogravimetric analysis, TGA, differential thermal analysis (DTA), and differential scanning calorimetry (DSC) are common thermal analyzes that are used to investigate the thermal stability of nanoparticles and the effect of temperature on nanoparticles. The results of these analyzes are shown in Fig. 9 for non-calcined WO3 nanoparticles. It is seen that a decrease in mass throughout the study period (15–1000 °C) occurred. Also, there are four reduction intervals in the curve. In the first interval (158–148 °C), the increase in the temperature has led to a decrease in the mass of the sample, which is due to the evaporation of moisture in the sample. Mass loss percent at 141.8 °C reaches 4.57%. The water liberation is an endothermic reaction, which is compatible with differential thermal analysis (DTA) and differential scanning calorimetry (DSC) data as shown in Fig. 9. The water removal process of WO3 nanoparticles at 140 °C has already been observed in the literature for tungsten oxides-based materials [32, 33]. The presence of a peak at 260 °C in the DTA plot confirms that an endothermic reaction has occurred in this region. This demonstrates that the second section of mass loss from 141.8 °C to 260 °C is due to the water removal from chemical bounds and it is an endothermic process. Mass loss at 260 °C reaches 9.2%. According to the DTA and heat flow plots, two exothermic processes have occurred in the third section of the TG plot (260–520 °C), which can be due to the change of the crystalline phase of the non-calcined WO3 nanoparticles. According to XRD results, the dominant crystal phase of NC and 300 T samples are orthorhombic and hexagonal, respectively. Therefore, the phase change in this section is confirmed by the XRD results. Also, according to XRD results, the 500 T sample is monoclinic, so another phase change is confirmed by XRD results in the section. Mass loss at 520 °C reaches 11.8%. In the range of 520–820 °C, mass loss is observed due to continuous crystal growth and the mass loss% reaches 1% at 860 °C. Also, the mass loss at >860 °C is due to the decomposition of the WO3 sample. Finally, the mass loss at the end of thermal analysis (1000 °C) reaches 14.6%.
3.6 Hydrodynamic diameter and colloidal stability
The colloidal stability and size distribution of nanostructures in the liquid medium is one of the important quantities that must be carefully considered in many applications such as water treatment. To investigate the stability and hydrodynamic diameter of the synthesized nanoparticles in the solution, a DLS measurement was carried out. The Zeta potential of the non-calcined and calcined samples at different temperatures of 300, 500, and 700 °C are shown in Fig. 10. The results show that the zeta potentials of the samples are located in the range of −26.6 to −23.0 mV, which means the nanostructures are in semi-stable conditions. Also, it is observed that the absolute value of zeta potential decreases with increasing the calcination temperature. Thus, the NC sample contains the most stable nanoparticles in the solution.
The size distributions of non-calcined and calcined WO3 nanoparticles are shown in Fig. 11. According to the results, the average dynamic size of non-calcined and calcined nanoparticles at temperatures of 300, 500, and 700 °C is 250.6, 358.2, 417.7, and 448.3 nm, respectively. As you can observe, the lowest dynamic size of nanoparticles belongs to the non-calcined WO3 nanoparticles, while the FESEM results reported the lowest particle size for the 300 T sample. This difference may be due to the better recognition of nanoparticles in DLS analysis, the better stability of non-calcined nanoparticles in the liquid, or the concentration and type of ions in the liquid medium.
Totally, the NC and 300 T samples show the smallest sizes and the highest liquid stability, and the best optical properties that are important for the interaction of laser light at 420 nm with the nanoparticles in liquid. Therefore, the interaction of laser light with these two WO3 nanostructures (NC and 300 T samples) are considered using degradation of MB solution as a famous dye and the photocatalytic activities of the samples are studied in the next section.
3.7 Photocatalytic activities of WO3 nanoparticles
ROS productions of NC and 300 T samples were examined by degrading MB (10 ppm) under exposing laser radiation (at 420 nm and power of 100 mW) in an acidic and neutral (pH = 5.4, 7.4) MB solution containing WO3 nanoparticles (0.1 mg of the NPs in 50 cc MB). The absorption spectra of acidic MB solution under treatment with irradiated non-calcined (NC sample) WO3 nanoparticles are depicted in Fig. 12a at different laser exposing times of 20, 40, 60, 80, 100, and 150 min, respectively. As you can observe, the decrement of absorption intensity over laser exposing time indicates the capability of as-synthesized WO3 nanoparticles for treatment of acidic water. Also, degradation% of MB solutions (with pH = 5.4, and 7.4) with irradiated non-calcined and calcined tungsten oxide nanoparticles (NC, and 300 T samples) at different laser exposing times is presented in Fig. 12b to study the photocatalytic activities of the selected WO3 nanoparticles in acidic and neutral conditions. The results declare that an increasing trend in MB degradation% over laser exposing time is observed in the samples, both in acidic and neutral conditions. However, the rate of the increase is not constant and varies for different samples as well as for different pH conditions. The degradation% of acidic MB (pH = 5.4) using irradiated non-calcined and calcined WO3 samples at the exposing laser time of 150 min is 74.7% and 44.4%, respectively, which confirms the high photocatalytic activities of non-calcined WO3 nanoparticles in acidic conditions. However, photocatalytic activities of non-calcined WO3 nanoparticles in acidic MB solution is higer than calcined one and it is higher than every reported photocatalytic efficiency of WO3 nanoparticles in acidic condition [12]. Also, The degradation% of neutral MB (pH = 7.4) using irradiated non-calcined WO3 samples at the exposing laser time of 150 min is 57.3%, which is an acceptable MB degradation%. Therefore, the non-calcined WO3 nanoparticles synthesized using sol–gel method at pH = 1 and aging time of 15 h can be suggested as an excellent candidate for water treatment and treatment of acidic effluents in factories. It can be concluded from the results that optical behavior and hydrodynamic size of particles play important roles for photocatalytic activities of the structure. However, better crystalline quality of NC sample compared to 300 T sample might be another reason for its better photocatalytic performance.
a Methylene blue absorption spectra after exposing a 405 nm laser into the acidic MB solution (pH = 5.4) containing 0.1 mg of non-calcined tungsten oxide nanoparticles at different laser exposing times of 10, 20, 30, 40, 50, 60, 80, 100, and 150 min and (b) degradation% of MB solutions (with pH = 5.4, and 7.4) under different laser exposing times into MB solution containing non-calcined or calcined tungsten oxide nanoparticles (NC, and 300 T samples)
4 Conclusion
water treatments are one of important global issues, which can be solved using photocatalytic activities of WO3 nanoparticles under exposure the light. The synthesis route and the synthesis parameters affect the nanoparticle’s properties including the morphology, crystallinity, stability, optical properties, and and photocatalytic activity. Therefore, WO3 nanoparticles were synthesized using sol–gel as a simple and low-cost method in a given condition and the nanoparticles were calcined at different temperatures (300, 500, and 700 °C) to find appropriate nanostructure for treatment of acidic water. Raman and FTIR spectra of all four samples have approximately similar peaks, which indicate the same chemical bonding configuration of the samples. The FTIR spectra and XRD results showed that the as-synthesized and calcined WO3 at 300 °C were hydrated, while calcined WO3 at 500 and 700 °C were non-hydrated. The XRD and thermal analysis point to a transition in the crystal phase with a change in calcination temperature. However, all the WO3 nanoparticles have strong x-ray diffraction peaks and therefore were crystalline nanoparticles. The highest crystallinity was observed for calcined WO3 nanoparticles at 700 °C. Also, the colloidal stability/hydrodynamic diameter of nanoparticles decreased/increased with increasing calcination temperature. The highest/lowest absorption/reflection in the range of 190–450 nm was obtained for non-calcined and calcined WO3 nanoparticles at 300 °C. These two samples showed the smallest particle size, the most stability in the liquid environment, and the best optical properties among other samples according to FESEM, DLS, and optical analyses. Therefore, the photocatalytic activities of non-calcined and calcined WO3 nanoparticles at 300 °C were investigated using degradation of MB solution under exposing the laser light to these nanoparticles. As-synthesized WO3 nanoparticles showed great photocatalytic activities in both neutral and acidic conditions. Also, unlike most nanoparticles, the photocatalytic efficiency of the non-calcined WO3 nanostructure in the acidic condition is noteworthy (74.7% in exposing time of 150 min), which makes it a suitable candidate for the treatment of acidic effluents in factories.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Aliannezhadi M, Minbashi M, Tuchin VV (2018) Effect of laser intensity and exposure time on photothermal therapy with nanoparticles heated by a 793-nm diode laser and tissue optical clearing. Quantum Electron 48(6):559
Jeevitha G, Abhinayaa R, Mangalaraj D, Ponpandian N (2018) Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J Phys Chem Solids 116:137–147
Elsayed EM, S Elnouby M, Gouda M, Elessawy NA, Santos D (2020) Effect of the morphology of tungsten oxide embedded in sodium alginate/polyvinylpyrrolidone composite beads on the photocatalytic degradation of methylene blue dye solution. Materials 13(8):1905
Han B, Popov A, Shekunova T, Kozlov D, Ivanova O, Rumyantsev A, Shcherbakov A, Popova N, Baranchikov A, Ivanov V (2019) Highly crystalline WO3 nanoparticles are nontoxic to stem cells and cancer cells. J Nanomater 2019:5384132
Zheng N, Zhang S, Wang L, Qi Z, Peng Q, Jian L, Bai Y, Feng Y, Shen J, Wang R (2022) Boosting image-guiding radiation therapy through W18O49 nanospheres and the second near-infrared light irradiation. Nano Res 15(3):2315–2323
Qureshi N, Lee S, Chaudhari R, Mane P, Pawar J, Chaudhari B, Shinde M, Rane S, Kim T, Amalnerkar D (2021) Hydrothermal Generation of 3-Dimensional WO3 Nanocubes, Nanobars and Nanobricks, Their Antimicrobial and Anticancer Properties. J Nanosci Nanotechnol 21(10):5337–5343
Rezaee O, Mahmoudi Chenari H, Ghodsi F (2016) Precipitation synthesis of tungsten oxide nanoparticles: X-ray line broadening analysis and photocatalytic efficiency study. J Sol-Gel Sci Technol 80(1):109–118
Yu J, Qi L, Cheng B, Zhao X (2008) Effect of calcination temperatures on microstructures and photocatalytic activity of tungsten trioxide hollow microspheres. J Hazard Mater 160(2-3):621–628
Derbasova N, Gavrish V, Oleynik A (2021) Studying the effect of monochrome light on the photocatalytic activity of tungsten oxide. J Phys Conf Ser 1:012117
Mohammed Harshulkhan S, Janaki K, Velraj G, Sakthi Ganapthy R, Nagarajan M (2016) Effect of Ag doping on structural, optical and photocatalytic activity of tungsten oxide (WO3) nanoparticles. J Mater Sci: Mater Electron 27(5):4744–4751
Khan MY, Ahmad M, Sadaf S, Iqbal S, Nawaz F, Iqbal J (2019) Visible light active indigo dye/graphene/WO3 nanocomposites with excellent photocatalytic activity. J Mater Res Technol 8(3):3261–3269
Deepa M, Kar M, Singh D, Srivastava A, Ahmad S (2008) Influence of polyethylene glycol template on microstructure and electrochromic properties of tungsten oxide. Sol Energy Mater Sol Cells 92(2):170–178
Ding R, Wang K, Hong K, Zhang Y, Cui Y (2019) Hierarchical core-shell tungsten oxide/TiO2 nanowires as an effective photocatalyst. Chem Phys Lett 714:156–159
Smrithi S, Kottam N, Arpitha V, Narula A, Anilkumar G, Subramanian K (2020) Tungsten oxide modified with carbon nanodots: Integrating adsorptive and photocatalytic functionalities for water remediation. J Sci: Adv Mater Devices 5(1):73–83
Sheikhi S, Aliannezhadi M, Tehrani FS (2022) Effect of precursor material, pH, and aging on ZnO nanoparticles synthesized by one-step sol–gel method for photodynamic and photocatalytic applications. Eur Phys J 137(1):60
Jamali M, Tehrani FS (2020) Effect of synthesis route on the structural and morphological properties of WO3 nanostructures. Mater Sci Semiconductor Process 107:104829
Ou P, Song F, Yang Y, Shao J, Hua Y, Yang S, Wang H, Luo Y, Liao J (2022) WO3· n H2O Crystals with Controllable Morphology/Phase and Their Optical Absorption Properties. ACS Omega 7(10):8833–8839
Jamali M, Tehrani FS (2021) Thermally stable WO3 nanostructure synthesized by hydrothermal method without using surfactant. Mater Sci Eng B 270:115221
Shariatmadar Tehrani F, Ahmadian H, Aliannezhadi M (2021) High specific surface area micro-mesoporous WO3 nanostructures synthesized with facile hydrothermal method. Eur Phys J 136(1):1–11
Tijani JO, Ugochukwu O, Fadipe L, Bankole M, Abdulkareem A, Roos W (2019) One-step green synthesis of WO3 nanoparticles using Spondias mombin aqueous extract: effect of solution pH and calcination temperature. Appl Phys A 125(3):1–12
Ahmadian H, Tehrani FS, Aliannezhadi M (2019) Hydrothermal synthesis and characterization of WO3 nanostructures: effects of capping agent and pH. Mater Res Express 6(10):105024
Abbaspoor M, Aliannezhadi M, Tehrani FS (2021) Effect of solution pH on as-synthesized and calcined WO3 nanoparticles synthesized using sol-gel method. Optical Mater 121:111552
Salmaoui S, Sediri F, Gharbi N, Perruchot C, Jouini M (2013) Hexagonal hydrated tungsten oxide nanomaterials: hydrothermal synthesis and electrochemical properties. Electrochim Acta 108:634–643
Tehrani FS, Ahmadian H, Aliannezhadi M (2020) Hydrothermal synthesis and characterization of WO3 nanostructures: Effect of reaction time. Mater Res Express 7(1):015911
Meng L, Zhao Z, Zhang M, Zhu X, Geng X, Liu J, Xia Y, Wang Z (2017) Synthesis of WO3 microfibers and their optical properties. Ceram Int 43(9):7048–7056
Shakya V, Pandey N, Misra SK, Roy A (2017) Electrical and optical properties of ZnO–WO3 nanocomposite and its application as a solid-state humidity sensor. Bull Mater Sci 40(2):253–262
Talukder A, Sultana P, Haider A, Wahadoszamen M, Abedin KM, Farhad S (2010) Power dependence of size of laser ablated colloidal silver nanoparticles. Eur Phys J D 60(2):295–300
Bahadori A, Dizaji HR, Memarian N, Aliannezhadi M (2020) Effect of preparation conditions on physical properties of manganese oxide thin films. J Sol-Gel Sci Technol 95(1):180–189
Gholizadeh Z, Aliannezhadi M, Ghominejad M, Tehrani FS (2022) High Specific Surface Area γ-Al2O3 Nanoparticles Synthesized by Facile and Low-cost Co-precipitation Method. preprint https://doi.org/10.21203/rs.3.rs-2085977/v1
Mioduska J, Zielińska-Jurek A, Janczarek M, Hupka J (2016) The effect of calcination temperature on structure and photocatalytic properties of WO3/TiO2 nanocomposites. J Nanomater 2016:3145912
Egorin A, Dran’kov A, Didenko N, Tokar E, Sokol’nitskaya T, Papynov E, Tananaev I (2020) Synthesis and sorption characteristics of tungsten oxides-based materials for Sr-90 removal from water media. J Mater Sci 55(22):9374–9384
Reis KP, Ramanan A, Whittingham MS (1992) Synthesis of novel compounds with the pyrochlore and hexagonal tungsten bronze structures. J Solid State Chem 96(1):31–47
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abbaspoor, M., Aliannezhadi, M. & Tehrani, F.S. High-performance photocatalytic WO3 nanoparticles for treatment of acidic wastewater. J Sol-Gel Sci Technol 105, 565–576 (2023). https://doi.org/10.1007/s10971-022-06002-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-06002-9