Abstract
This study reports the humidity sensing characteristics of ZnO–WO3 nanocomposite. Pellet samples of 0–5 weight% ZnO in WO3 were sintered from 300 to 600∘C. When exposed to humidity, the resistance of the sensing samples was found to decrease with increase in relative humidity (RH). Five percent ZnO-doped WO3 showed maximum sensitivity of 20.95 M Ω/%RH in 15–95% RH range. Sensor parameters like reproducibility, aging, hysteresis, response and recovery times were also studied. Sensing mechanism is discussed in terms of sintering temperature, composition and crystallite size of the sensing element. It was observed that sensing mechanism is strongly based on annealing temperature and percentage of doping. The sensing samples have also been investigated by X-ray diffraction, scanning electron microscope (SEM) and Raman spectroscopy. The crystalline size of the sample was identified by powder X-Ray Diffraction data. The SEM analysis was used to study the surface morphology. The structure, phase and the degree of crystallinity of the materials were examined by Raman spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Humidity monitoring and controlling devices have significant importance in industrial, environmental, agricultural and medical fields. Resistive humidity sensors are the devices that transduce humidity change to resistance change. Three groups of materials have been used and reported for resistive humidity measurements: ceramics, polymers and electrolytes. Ceramics such as WO3, ZnO, SnO2, Al2 O 3, TiO2, etc. are most investigated sensing materials for relative humidity (RH) sensors. Nowadays, varieties of humidity sensor that are available or under review process for development in various research institutions are RH-type sensors. We can categorize these type of sensors into three classes, including ceramic type (semiconductor), organic polymer-based sensors and organic/inorganic hybrid sensors (polymer/ ceramic). Humidity sensors that are currently manufactured by using nanowires, nanofibres, nanorods and p–n heterojunctions are subclasses of the ceramic (inorganic) type.
The intrinsic characteristics of ceramic material namely, reproducibility of the electrical properties, mechanical strength, chemical and physical stability have been a centre of attraction nowadays. These type of materials possess a unique structure consisting of grains, grain boundary surfaces and pores, which make them suitable for adsorption of water molecules because of the high surface exposure [1]. Generally, metal oxides can be classified into two types, non-transition and transition; the binary transition-metal oxides (e.g., TiO2, V2 O 5, WO3) with d0 configuration and post-transition oxides (e.g., ZnO, SnO2) with d10 configuration. Transition-metal oxides behave differently because the energy difference between a cation dn configuration and either a dn+1 or dn−1 configurations is often rather small [2]. Due to small energy differences the transition metal oxides are utilized for sensing applications, because they can form various oxidation states on the surface of metallic oxides.
Tungsten oxides (WO3) are a strong candidate used for sensing purpose, with high sensitivity and also long-term stability [3]. Consequently, this system has been examined in substantial detail using several categories of material like bulk ceramic [4], thin film [5–8] and thick film [9]. Tungsten trioxide (WO3) has been investigated intensively because of its intriguing structures, physical and chemical properties [10–13], and potential applications [14–18]. One of the elementary defects in tungsten oxide structure, as in most metal oxide, is the lattice oxygen vacancy, where an oxygen atom is lacking from the normal lattice site. The octahedral variation in corners sharing to edge sharing lattice with the formation of crystallographic shear planes built up of edges and plane sharing structures, which corresponds to particle replacement of W6+ to W5+ and W4+ ions. This leads to the formation of non-stoichiometric tungsten oxides WO3−x and strongly influences its electronic properties [19]. ZnO is an attractive semiconductor oxide for sensing purpose having a wide bandgap and is known for its n-type conduction due to the presence of oxygen vacancies [20,21]. It is observed that the sensitivity of pure sensing material increases after doping of ZnO because the amount of highest carrier concentration of holes and electrons attained to about 1019 and 1020 cm−3, respectively, were reported [22].
Dewyani Patil et al[23] synthesized poly(2, 5-dimethoxyaniline)/WO3 (PDMA/WO3) composites by mechanical mixing method and investigated the humidity sensing properties. The composite with 30 weight% of WO3 changes linearly over the humidity range 23–84% and RH shows maximum percentage response factor (∼651 at 87% RH), quick response (humidification, 27 s and desiccation, 136 s), narrow hysteresis (∼5%) and an excellent repeatability of the response [23]. Yadav et al[24] reported the synthesis and characterization of ZnO–TiO2 nanocomposite and its application as humidity sensor. ZnO was prepared by conventional precipitation method using sodium hydroxide. The ZnO–TiO2 nanocomposite exhibits its highest average sensitivity of 18 M Ω/%RH over the entire range of %RH, i.e., from 10 to 95% RH at room temperature (19∘C). The average size of nanoparticles is found to be a minimum in the range 50–200 nm at 19∘C temperature.
Sundaram and Nagaraja [25] report that lead (II) tungstate and zinc (II) tungstate were prepared by a solution route method and sintered at 973 K in the form of cylindrical discs. The composites were subjected to d.c. resistance measurements as a function of RH in the range 5–98% RH, achieved by different water vapour buffers thermostated at room temperature. The composites have the highest humidity sensitivity factor of 17615 ± 3000 and 2666 ±550, respectively. The response and recovery time for these humidity sensing composites were good. Noubeil Guermat et al[26] report the study of humidity-sensitive and electrical properties of plasma polymerization of hexamethyldisiloxane (pp-HMDSO) thin film-based sensors. The humidity sensitive film was deposited by glow discharge at low frequency power (19 kHz) in a capacitive-coupled parallel plate plasma reactor. The deposited film sensor exhibited a small hysteresis (2% RH) and fast response (8 and 34 s for adsorption and desorption between 35 and 95% RH, respectively). Md Sin et al[27] have reported about the electrical characteristics of aluminium (Al)-doped zinc oxide (ZnO) thin film for high sensitivity humidity sensors. Parameter 0.6 at% aluminium-doped ZnO showed high sensitivity of 2.32 M Ω/%RH and suitable for humidity sensor. Photoluminescence showed emissions band with two peaks centred at about 380 (ultra-violet (UV)) and 600 nm (green) at room temperature (table 1).
The above developments inspired us to prepare suitable material for the humidity sensing applications with improved sensitivity. In our earlier study, we have reported the morphological and RH sensing studies of pure WO3 and WO3–ZnO (samples WZ-10, WZ-25 and WZ-50) nanocomposite prepared by solid-state reaction route sintered in air at temperature 300–500∘C [34]. The present paper reports the electrical and optical properties of ZnO–WO3 (samples ZW-1, ZW-2, ZW-3, ZW-4 and WZ-5) nanocomposite and its application as a solid-state humidity sensor sintered in air at temperature 300–600∘C. The aim of this study is to examine the variation induced on the sensing element by ZnO doping. The nature of the changes analysed is two-fold. On the one hand, morphological and crystalline variations are studied and, on the other hand, variations in response to humidity are investigated. Scanning electron microscopy (SEM), X-ray diffraction (XRD) and Raman spectra have been used to study the morphological variation, to investigate the changes in the crystalline structure and to examine the structure, phase and components of materials, respectively. The sensitivity in present case is higher for ZW-5 (20.95 M Ω/%RH) than the sensing element WZ-50 (1.20 M Ω/%RH) in earlier case. The present study also indicates that there is an improvement in the sensor performance in terms of repeatability and hysteresis. A positive humidity sensing phenomenon was observed between pure WO3 and ZnO-doped WO3, which might explain the doping and temperature dependency of the sensing material that was not found in the earlier case.
2 Sample preparation and experimental process
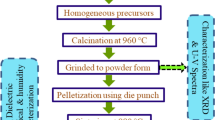
The nanocomposite sample of ZnO–WO3 was prepared through solid-state reaction route. The starting materials taken were WO3 (Loba Chemie, 99.99% pure) and 1 weight% (sample ZW-1), 2 weight% (sample ZW-2), 3 weight% (sample ZW-3), 4 weight% (sample ZW-4) and 5 weight% (sample ZW-5) of ZnO powder (Loba Chemie, 99.9%) added in WO3. Polyvinyl alcohol (10% weight) is added as binder to increase the strength of the sample. Mixed powder was ground to uniformity for 3 h. Then the resultant powder was pressed into pellet shape by uniaxially applying pressure of 267 MPa in a hydraulic press machine (M.B. Instruments, Delhi, India) at room temperature. The prepared pellet samples were in disc shape, having a diameter of 15 mm and 2.3 mm in thickness. Pressed powder pellets were sintered in air at 300–600∘C for 3 h in an electric muffle furnace (Ambassador, India) and cooled to room temperature. After sintering, each sensing sample was placed in the same humidity chamber and its sensing outputs were obtained under different humidity levels. Figure 1 shows the schematic diagram of humidity sensing apparatus. Saturated salt solution of potassium sulphate was used as a humidifier and potassium hydroxide as de-humidifier. Inside the humidity chamber, a thermometer (±1∘C) and standard hygrometer (±1% RH, digital) were placed for the purpose of calibration. Electrical characteristic of the sensors was measured by a multifunctional digital multimeter (±0.001 M Ω, VC-9808) at variant humidity environments. The electrical resistance of the sensing elements in the form of pellets have been measured normal to the cylindrical surfaces of the pellets at different RH levels by two-probe method. The electrical contacts were made on the surface of pellets by means of two thin copper sheets. Given high resistivity of materials under consideration, potential inaccuracy due to contact resistance was assumed negligible. The surface contact area of all sensing elements with electrodes was 113.11 mm2 and the cylindrical surface area that was exposed to the humidity in the chamber was also 113.11 mm2. These values were kept constant for all the sensing elements. Experimental samples were electrically connected to a power supply and resistance metre in series. After studying humidity sensing properties, sensing elements were kept in laboratory environment and their humidity sensing characteristics were regularly monitored. To see the effect of aging, sensing properties of these elements were examined again in the humidity chamber after 8 months. Stability of sensing elements was checked by keeping the sensing element at fixed values of %RH in the chamber and the values of resistance were recorded as function of time. Samples of pure WO3 (0% ZnO-doped WO3), 1% ZnO-doped WO3, 2% ZnO-doped WO3, 3% ZnO-doped WO3, 4% ZnO-doped WO3 and 5% ZnO-doped WO3 have been labelled ZW-0, ZW-1, ZW-2, ZW-3, ZW-4 and ZW-5, respectively.
3 Principle of operation
In ceramic sensors, resistance of sensor decreases with increase in RH due to physisorption and capillary condensation of water molecules on the surface of the material (figure 2). Water molecules act as electron donating gas and their chemisorption increases or decreases electronic conductivity depending on whether the material is n- or p-type semiconductor. When moisture comes in contact with ZnO–WO3 nanocomposite, water molecules irreversibly chemisorb on oxide surface [35–37]. The chemisorbed layer can be thermally removed by increasing the surrounding temperature. For transition-metal ion M+ the irreversible reaction for the first layer may be written as
Subsequent water layers are physically adsorbed on the first chemisorbed layer. The physisorbed water layer is bound by weak hydrogen bonding on the chemisorbed layer and may be removed by decreasing humidity. The chemisorbed water molecule attracts and breaks oxygen to hydrogen bonds of physisorbed water molecules by following mechanism proposed by Friot et al[38] and Anderson and Parks [39]:
This physisorbed layer contributes mostly to humidity sensitive conduction of metal oxide ceramics leading to the conduction of electrons at the pure WO3 or ZnO–WO3 nanocomposite surface that causes decrease in resistance with increase in RH.
4 Results and discussion
4.1 Humidification graphs
Figure 3 shows the humidification graphs for the sensing samples ZW-0, ZW-1, ZW-2, ZW-3, ZW-4 and ZW-5 for the sintering temperature 600∘C in terms of resistance vs. RH at room temperature repeated over many cycles. Figure 3 shows large decrease in the value of the resistance against initial values of RH from 15 to 40% RH, while in 40–95% RH range the decrease in resistance is slow. The maximum sensitivity of 20.95 M Ω/%RH has been found for the sensing element ZW-5 as compared to the sensitivity of the sensing elements ZW-0, ZW-1, ZW-2, ZW-3 and ZW-4. As observed from figure 3, the value of sensitivity increases with increase in the percentage of ZnO in WO3.
Higher porosity increases surface-to-volume ratio of the materials and enhances diffusion rate of water into or out-off the porous structure; thus, at high humidity (> 40% RH) the proton hopping and the ionic conduction increase surface conductivity while at low humidity (< 40% RH) there is no effective protonic conduction [40]. Overall, the resistance of the ceramic decreases with an increase in humidity [41]. The decrement in resistance or increase of conductance also occurs due to the variation of grain boundary barrier height inside the ceramics. In this case the adsorption of water molecules interpenetrated at WO3 surface can decrease the height of potential barriers at the surface of WO3 grains and at the surface of necks between WO3 grains. Therefore the size of the depletion regions in the vicinity of the necks in the direction of electric field is lowered and conductance of ceramics is increased. Penetrated water molecules can promote the decrease in the barrier heights of grain boundary potential barriers exponentially; therefore slight decrease in grain boundary barriers height on RH can cause strong decrease in resistance (or increase of conductance). This is schematically shown in figure 4. In this case the conductance can change with the value of RH exponentially [42].
Sensitivity of humidity sensor is defined as the change in resistance (ΔR) of sensing element per unit change in RH (ΔRH%). For calculation of sensitivity, humidity from 15 to 95% RH was divided in equal intervals of 5% RH each. The difference in the value of the resistance for each of this interval was calculated and then divided by 5. An average was taken for all these calculated values. Formula for calculation of sensitivity of the sensing elements may be written as given below:
Sensitivity values for the sensing samples ZW-0, ZW-1, ZW-2, ZW-3, ZW-4 and ZW-5 for the annealing temperature 600∘C have been calculated and shown in tables 2 and 3. The average sensitivity for the sensing sample WZ-0, ZW-1, ZW-2, ZW-3 and ZW-4 is lower compared to that of sample ZW-5 at the sintering temperature 600∘C. As the sintering temperature increases from 300 to 600∘C, the sensitivity of the sensing sample ZW-5 increases (table 3). The sample ZW-5 shows results with maximum sensitivity of 12.62, 16.25, 17.12 and 20.95 M Ω/%RH among all samples when annealed at 300, 400, 500 and 600∘C, respectively, as shown in figure 5.
4.2 Humidification and desiccation graphs (hysteresis)
To determine the hysteresis effect in the sensing elements, first humidity was increased from 15% RH to 95% RH and then cycled down to 15% RH in the humidity chamber, and the values of resistance of the sensing elements were recorded against the variation of %RH. Figure 6 shows hysteresis graph for the sensing element ZW-5 for sintering temperature 600∘C. All sensing elements have acceptable hysteresis values. The change in the value of sensitivity in decreasing cycles over increasing cycles for samples ZW-0, ZW-1, ZW-2, ZW-3 and ZW-4 were ±0.10%, ±3.12%, ±2.85%, ±2.71% and ±1.04%, respectively, for the sintering temperature 600∘C. Our best sensing element ZW-5 have an acceptable hysteresis values ±1.78%, ±1.63%, ±0.92% and ±0.52% for sintering temperatures 300–600∘C. Data sheets of some commercially available humidity sensors indicates hysteresis values in the range from 1.20 to 5.00% [43,44]. The phenomenon of hysteresis may be understood in the manner that due to the adsorption of water on the surface of the sensing element a chemisorbed layer is formed. This chemisorbed layer, once formed is not further affected by exposure to or removal of humidity, it can only be thermally desorbed. Hence, in the decreasing cycle of %RH, the initially adsorbed water is not removed fully, leading to hysteresis.
Thus, the maximum change in the values of sensitivity for the decreasing cycles over increasing cycles for sensing sample WZ-0, WZ-1, WZ-2, WZ-3 and WZ-4 is within the range 0.1–3.2% for the sintering temperature 600∘C, while for the sensing sample ZW-5 it is within the range 0.5–1.8% for the sintering temperatures 300–600∘C.
4.3 Ageing effect
Ageing is a significant problem in sensing devices based on metal oxides. To see the aging effect, the sensing elements were kept in laboratory environment and their humidity sensing characteristics were regularly monitored. The sensing properties of the samples were examined again in the humidity chamber after 4 and 8 months and the variation of resistance with %RH recorded. Here, % ageing has been defined as the % deviation in the value of sensitivity over a period of time (for example, 2 months, 8 months, etc.) from the initial measured values. This may be termed repeatability over a period of time as well. The repeatability graph for the sensing element ZW-5 with change in %RH after 4 and 8 months have been shown in figure 7. Corresponding values of sensitivity after 4 and 8 months for sensing elements ZW-0, ZW-1, ZW-2, ZW-3, ZW-4 and ZW-5 are shown in tables 2 and 3. In figure 7, both an initial increasing cycle and another increasing cycle after 4 and 8 months have been shown. We observe that for the sensing sample ZW-5, there is large improvement in reproducibility after 4 and 8 months for the sintering temperature 600∘C. Ageing effect present in the ceramic humidity sensor may be due to either prolonged exposure of surface to high humidity, adsorption of contaminants preferentially on the cation sites, loss of surface cations due to vaporization, solubility and diffusion, or sintering to a less reactive structure, migration of cations away from the surface due to thermal diffusion [45,46].
4.4 Regression analysis
Regression analysis of the data was carried out on humidification graphs. Least square fit with respect to polynomials of third degree was established. The coefficients of various power of x (RH) with respect to y (Resistance) are given in table 4. From polynomial of degree 3, value of coefficients of the first power of x are given in table 4; i.e., a 1 is the dominant one, which represents linearity between x and y (RH and resistance). Considering the present state of instrumentation, it is not essential to go for a linear response in sensors; a single valued function should suffice. The nanocomposite qualifies to be a good ceramic humidity sensor with the polynomials of third degree.
4.5 Response and recovery time
Time taken to accomplish 90% of the initial total resistance variation is defined as response/recovery time during the humidification and desiccation processes. To find the response and recovery time, the graph shows the variation of resistance with time for sample WZ-5, i.e., the best sample. For sample WZ-5, the response time was 85 s when RH was changed from 15 to 95%, while the recovery time was 550 s when RH was changed from 95 to 15% as shown in figure 8. Adsorption and desorption of the water molecules take place at different energy levels. Adsorption is an exothermic process, where as desorption needs external energy for water molecules to depart from the metal oxide surface. As desorption is an endothermic process, it takes a longer time to desorb the water vapour; therefore, the recovery time is always greater than the response time [47–49].
4.6 XRD analysis
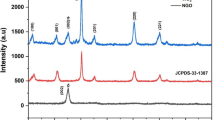
XRD has been studied using XPERT PRO-Analytical XRD system (The Netherlands). The wavelength of the source CuK α used is 1.54060 Å. Figure 9 shows the XRD pattern of sensing element WZ-5 annealed at temperature 600∘C. The XRD pattern for the sensing element WZ-5 annealed at 600∘C shows a lot of WO3 peaks. High intensity, large number of peaks and sharpness of peaks show high crystallinity of the sensing element WZ-5 annealed at 600∘C [50,51]. Some peaks are found broad and the broadness of the peak indicates that crystallites are of small size or nano-size. From Scherer’s formula we can see that broader the diffraction peak (i.e., greater the full-width at half-maximum (FWHM) value) smaller the crystal size. XRD pattern for sample ZW-5 shows the monoclinic structure of WO3 with space group P21/n and the hexagonal structure of ZnO with space group P63mc. Major phase of WO3 exists for 2 𝜃 = 24.71∘ at the plane (200) and d-spacing corresponding to this peak is 3.60000 Å (figure 9). Scherer’s formula gives measurement of crystallite size in sample. Average crystallite size of the samples was calculated using Debye Scherer’s formula:
where D is the crystallite size, K the fixed number of 0.9, λ the X-ray wavelength, 𝜃 the Bragg angle and β FWHM of the peak. The minimum crystallite size calculated from Scherer’s formula for the sample ZW-5 is found to be 16 nm. Average crystallite size of sample WZ-5 annealed at 600∘C is found to be 48 nm. These crystallites may not be the only factor that affects sensitivity. Crystallites in metal oxides have tendency to agglomerate and form larger grains. The measurement of grain size by SEM for different sintering temperatures was carried out.
4.7 SEM study
To study the grain micro-structure, SEM micrographs were taken, which would provide a view of the grain development and grain size. SEM micrographs show that porous structure is dependent on the sintering temperature. Sintering at each temperature gives characteristic typical porous structure and small crystallites without inner pores but many intergrain pores. In addition, it can be observed that intergranular pores are linked through the large pores. The pore structures should be regarded as interconnected voids that form a kind of capillary tube. This structure favours adsorption and condensation of water vapour. Figure 10 shows SEM micrographs of sensing element ZW-5 sintered at temperatures 300, 400, 500 and 600∘C. The micrographs were taken at 35.00 KX magnifications. SEM micrographs show that the sensing elements manifest porous structure having granulation and tendency to agglomerate. SEM image, figure 10a, of sensing sample WZ-5 for the sintering temperature 300∘C shows highly densely interconnected clusters of small grains (120–180 nm), while figure 10b shows the formation of highly interconnected clusters of small grains (120–240 nm) and densely packed aggregates in sensing sample WZ-5 for the sintering temperature 400∘C. When sintering temperature is changed from 400 to 500∘C, these dense aggregates grow rapidly and homogeneous grains form (180–240 nm in size) with intergranular pores and capillary pores (figure 10c). At higher temperature 600∘C, shown in figure 10d, these grains still grow, such that they form large grains (300–540 nm in size) with larger intragranular pores and a network of capillary pores, which is expected to provide sites for adsorption. As sintering temperature increases, crystallinity of the sensing sample increases and the porosity increases surface-to-volume ratio of the material. Due to the presence of larger grains, more of the surface areas of the sensing elements are exposed leading to more adsorption of water molecules. This increases the sensitivity of the sensing elements.
Arresting particle growth by additives is an established ceramic technique. In general, when the additive ions are not accommodated in the lattice, they tend to segregate at the grain boundaries of host particles. Zn2+ having much bigger size (88 pm) than W6+ (67 pm) or W4+ (68 pm) is not expected to take lattice site. Therefore, it is reasonable to suggest that only a part of the Zn2+ takes lattice site for all the compositions, while the remaining part gets segregated at the WO3 grain boundaries, thus arresting particle growth [52]. With increase in sintering temperature this agglomeration around the host ions increases leading to grain size growth. For sample WZ-5, average grain sizes are 132, 180, 200 and 348 nm for sintering temperatures 300, 400, 500 and 600∘C, respectively. As sintering temperature is increased, an evolution in the distribution of the grain size occurs, and that the crystallinity increases with increase in the sintering temperature. The texture and grain size distribution are highly influenced by the increase in sintering temperature. More voids are created during the sintering process. Scanning electron micrographs for sintering temperatures 400 and 500∘C manifest a continuity and almost uniform distribution of grains, increasing the formation of voids and interconnected voids or capillaries which are very important conditions for the adsorption of water molecules in the sensing elements. Thus, they will show increasing adsorption capacity for moisture and hence the increasing sensitivity. Sensing element sintered at 600∘C has the highest crystallinity as well as the maximum void concentration and porosity. This synergy turns out to be the reason for the affinity towards moisture and hence a higher conductivity. It may be argued that the higher sensitivity may be due to a multiplicative effect of two or more than two parameters of the resultant composite. Samples sintered at lower temperatures may have more grains per unit area of the surface, yet the range in size of grain is so narrow that the surface of the composite appears smooth and therefore does not provide kinks as adsorption sites [53]. Higher porosity increases surface-to-volume ratio of the materials and therefore, helps in getting good sensitivity. It also helps in reducing response and recovery times because it enhances diffusion rate of water into or out-off the porous structure. An observation of the crystallite size and grain size would suggest that smaller crystallites are getting agglomerated to form larger grains. In this process, more of the surface areas of the sensing elements are exposed leading to more adsorption of water molecules. This increases the sensitivity of the sensing elements.
4.8 Raman spectroscopy
Raman scattering is a very sensible tool to examine the structure, phase and components of materials such as tungsten oxides. It is suitable to study the vibration and rotation of molecules. As shown in figure 11, the Raman spectrum of the pure WO3 and ZnO-doped WO3 nanostructures sintered at 600∘C exhibits five Raman peaks placed at around 134, 275, 327, 715 and 803 cm−1. In Raman spectrum, peaks in the 700–803 cm−1 range correspond to the stretching vibrations of W–O bonds while the bands at 275, 327 and lower 200 cm−1 correspond to the deformation vibrations of O–W–O bonds, O–W–O bending modes of the bridging oxygen and lattice modes [54]. The peaks at 803 and 715 cm−1 are Raman peaks of crystalline WO3 (m-phase), which correspond to the stretching vibrations of the bridging oxygen [55]. These peaks are attributed to W–O stretching (ν), W–O bending (δ) and O–W–O deformation (γ) modes [56,57]. Figure 11 shows absent of substantial peaks, which means no impurities were present in the samples. The Raman spectra of crystalline WO3 (WO3 crystal does not have any double bond) [58,59]as well as all the above information confirms the hypothesis of an open (or porous) structure of the sample with many inner empty spaces and intergrain boundaries. This means the sample is suitable for sensing purpose.
5 Conclusion
Pure WO3 and composites with different % doping of ZnO in WO3 were fabricated and studied for humidity sensing applications. The SEM revealed that WZ-5 composites at annealing temperature 600∘C have higher porosity, which increases surface-to-volume ratio of the materials and therefore, helps in getting good sensitivity. It also helps in reducing response and recovery time because it enhances diffusion rate of water into or out-off the porous structure, hence are good candidates for humidity sensors. Among all composites 5 wt% of ZnO–WO3 composite shows high sensitivity, lower hysteresis and less effect of ageing. Hence our studies suggest that, ZnO–WO3 composite can be a promising material for high-performance humidity sensing applications.
References
Pelino M. and Cantalini C. 1994 Active and Passive Elec., Comp. 16 69
Wang C, Yin L, Zhang L, Xiang D and Gao R 2010 Sensors 10 2088
Akiyama M, Tamaki J, Miura N and Yamazou N 1991 Chem. Lett. 9 1611
Inoue T, Ohtsuka K, Yoshida Y, Matsuura Y and Kajiyama Y 1995 Sens. Actuators B 25 388
Cantalini C, Sun H T, Faccio M, Pelino M, Santucci S, Lozzi L and Passacantando M 1996 Sens. Actuators B 31 81
Sberveglieri G, Depero L and Groppelli S 1995 Sens. Actuators B 26 89
Cantalini C, Pelino M, Sun H T, Faccio M, Santucci S, Lozzi L and Passacantando M 1996 Sens. Actuators B 35 112
Lee D S, Lim J W, Lee S M, Huh J S and Lee D D 2000 Sens. Actuators B 64 31
Chung Y K, Kim M H, Um W S, Lee H S, Song J K, Choi S C, Yi K M, Lee M J and Chung K W 1999 Sens. Actuators B 60 49
Turyan I, Krasovec U O, Orel B, Saraidorov T, Reisfeld R and Mandler D 2000 Adv. Mater. 12 330
Ingham B, Hendy S C, Chong S V and Tallon J L 2005 Phys. Rev. B 72 075109
Kofstad P 1972 Nonstoichiometry, diffusion, and electrical conductivity in binary metal oxides (New York: Wiley) p 208
Berak J M and Sienko M J 1970 J. Solid State Chem. 2 109
Yang R, Terabe K, Liu G Q, Tsuruoka T, Hasegawa T, Gimzewski J K and Aono M 2012 ACS Nano 6 9515
Chang T, Jo S H and Lu W 2011 ACS Nano 5 7669
Yang R, Terabe K, Yao Y P, Tsuruoka T, Hasegawa T, Gimzewski J K and Aono M 2013 Nanotechnology 24 384003
Zhao M, Huang J X and Ong C W 2012 Nanotechnology 23 315503
Dai C L, Liu M C, Chen F S, Wu C C and Chang M W 2007 Sens. Actuators B 123 896
Jimenez I, Arbiol J, Dezanneau G, Cornet A and Moronte J R 2003 Sens. Actuators B Chem. 93 475
Kishimoto S, Yamamoto T and Nakagawa Y 2006 Super Lattices Microstruct. 39 306
Yogeeswaran G, Chenthamarakshan C R, Tacconi N R and Rajeshwar K 2006 Mater. Res. Soc. 21 3234
Seghier D and Gislason H P 2008 J. Mater. Sci. Mater. Electron. 19 687
Patil D., Seo Y. -K., Hwang Y. K., Chang J. -S. and Patil P. 2008, Sens. Actuators B 132 116
Yadav B C, Srivastava R. and Dwivedi C D 2008 Philosophical Magazine 88 113
Sundaram R and Nagaraja K S 2004 Mater. Res. Bull. 39 581
Guermat N., Bellel A., Sahli S. and Raynaud P. 2014, J. Chem. Sci. Technol. 3 13
Md Sin N D, Fuad Kamel M, Alip R. I., Mohamad Z. and Rusop M. 2011, Adv. Mater. Sci. Eng. 2011 5
Srivastava R. and Yadav B C 2012 Adv. Mat. Lett. 3 197
Pandey N K, Tiwari K and Akash R O Y 2012 Bull. Mater. Sci. 35 347
Li H., Liu B., Cai D., Wang Y., Liu Y., Mei L., Wang L., Wang D., Li Q. and Wang T. 2014, J. Mater. Chem. A 2 6854
Pandey N K and Tiwari Karunesh 2010, Sensors Trans. 122 9
Pandey N K, Tiwari K. and Roy A. 2011, IEEE Sens. J. 11 2911
Pandey N K, Tiwari K. and Roy A. 2011, IEEE Sens. J. 11 2142
Pandey N K, Tripathi A, Tiwari K, Roy A, Rai A, Awasthi P, Mishra A and Kumar A 2008 Sensors Trans. 96 42
Fleming W J 1981 Society of automotive engineers transactions 90 1656
Seiyama T, Yamazoe N and Arai H 1983 Sens. Actuators 4 85
Yadav B C, Pandey N K, Srivastava Amit K and Sharma Preeti J 2007 Meas. Sci. Technol. 260 18
Frioat J J, Jelli A, Poncele G and André J 1965 J. Phys. Chem. 69 2185
Anderson J H and Parks G A 1968 J. Phys. Chem. 72 3662
Varghese O K and Grimes C A 2003 J. Nanosci. Nanotechnol. 3 277
Qu W and Ray Green M 2000 Austin, Meas., Sci. Technol. 11 1111
Skuratovsky I, Glot A, Di Bartolomeo E, Traversa E and Polini R 2004 J. Eur. Ceram. Soc. 24 2597
Thermoset polymer-based capacitive sensors, application sheet (Online). Available: www.honeywell.com
HIH-3605 Humidity sensor data sheet (Online). Available: www.honeywell.com
Ohbuchi Y, Kawahara T, Okamoto Y and Morimoto J 2001 J. Appl. Phys. 40 213
Saha D, Giri R, Mistry K K and Sengupta K 2004 Sens. Actuators B Chem. 107 323
Saha D, Giri R, Mistry K K and Sengupta K, Sens. Actuators B Chem. 107 323
Qu W and Meyer J -U 1997, Sens. Actuators B 40 175
Sundaram R, Raj E S and Nagaraja K S 2004 Sens. Actuators B 99 350
Rout C S, Hegde M and Rao C N R 2008 Sens. Actuators B 128 488
Na D. -m., Satyanarayana L., Choi G. -P., Shin Y. -J. and Park J. S. 2005, Sensors 5 419
Kim H. -K., Sathaye S. D., Hwang Y. K., Jhung S. H., Hwang J. -S., Kwon S. H., Park S. -E. and Chang J. -S. 2005, Bull. Korean Chem. Soc. 1881 26
Pandey N K, Tiwari K, Roy A, Mishra A. and Govindan A. 2012, Int. J. Appl. Ceram. Technol. 10 150
Lethy K J, Beena D, Kumar R V, Pillai V P M, Ganesan V, Sathe V and Phase D M 2008 Appl. Phys. A Mater. Sci. Process 91 637
Tagtstrom P and Jansson U 1999 Thin Solid Films 352 107
Daniel F, Desbat B, Lassegues J C, Gerand B and Figlarz M 1987 J. Solid State Chem. 67 235
Shigesato Y, Hayashi Y, Masui A and Haranou T 1991 Jpn. J. Appl. Phys. 30 814
Dixit L, Kapoor S K, Singh I D and Gupta P L 1977 Indian J. Phys. B51 116
Daniel M F, Desbat B, Lassegues J C and Garie R 1988 J. Solid State Chem. 73 127
Acknowledgements
We would like to thank the University Grants Commission, India, for providing the financial support for carrying out the research work through Major Research Project Grant [(file no. 42-788-2013 (SR)]. Thanks are also due for the Geological Survey of India, Lucknow, for providing XRD facility and Birbal Sahni Institute of Paleobotany, Lucknow, for providing SEM facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shakya, V., Pandey, N.K., Misra, S.K. et al. Electrical and optical properties of ZnO–WO3 nanocomposite and its application as a solid-state humidity sensor. Bull Mater Sci 40, 253–262 (2017). https://doi.org/10.1007/s12034-017-1373-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-017-1373-5