Abstract
Herein report, we aim to study the structural, dielectric, and magnetic properties for multifunctional perovskite materials LaCo0.2Mn0.8O3 and La2CoMnO6 nanoparticles, which were synthesized by a modified sol–gel route. Citric acid was processed as the chelating agent; gelation slurry formed by irradiation process which exposed to a total gamma radiation dose of 25 kGy at a dose rate of ~1.2 Gy/h to obtain more stability and high purity multifunctional perovskite materials. XRD notarizes the genesis of one pure phase orthorhombic perovskite structure. A full agreement between the particle sizes was investigated by HRTEM and the XRD data was observed. Raman spectra result assigned to the antisymmetric stretching mode and symmetric stretching mode of the (Co/Mn) O6 octahedra, which related to lattice distortions. The as-prepared perovskite materials exhibit ferromagnetic nature with different values of magnetization. Electron Spin Resonance (ESR) measurements are also carried out in the LCMO multifunctional nanoparticle systems, which suggests the occurrence of Jahn–Teller glass analogous to the spin-glass behavior. The complex impedance displayed high impacts on the electrical properties. The high value of dielectric constant for LCMO prepared nanoparticle systems may be used in electric tunable devices.

Highlights
-
Novel modified sol-gel route by gamma radiation used to synthesis more stability and high purity multifunctional perovskite materials (LaCo0.2Mn0.8O3, and La2CoMnO6).
-
These multifunctional materials have orthorhombic structure lattices.
-
LCMO multifunctional nanoparticle systems have a spin-glass behavior.
-
The high value of dielectric constant for LCMO nanoparticle multifunctional materials is promising in electric tunable devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multifunctional materials with perovskite category structures possess expanded physical properties such as dielectric, ferroelectric, ferro-/antiferromagnetic, and electronic/ionic conductions to use for the electric prospective applications. Such types from these materials are used in solar cells, electric tunable devices, biomolecular, and gas sensing technologies, and spintronics and magneto-electric devices [1].
In the AMO3 perovskite, A = rare-earth (where, A = La, Nd, Sm, Gd, and Pr), M = transition metal ions (like, Mn, Co, Fe, etc.), while A2MM’O6 double perovskite (A = rare-earth or alkaline earth ions; M and M’ = 3d transition metal ions) are a type of perovskites which have represented noteworthy attention [2,3,4,5,6]. The characteristic property of an ordered rock-salt is the arrangement of corner-sharing MO6 and M′6 units in the crystal structure. As a result of alternative composition in structure for double perovskite materials, a vast diversity of physical properties in DPs (Double Perovskite) is exhibited [7]. Strong conjunction between the magnetic and dielectric properties in some multifunctional double perovskite systems with the cohabitation of insulating and ferromagnetic properties has acquired significant research interest. Lanthanum-based perovskite-type oxides (such as LCMO (Lanthanum Cobalt Manganese oxide), and LNMO (Lanthanum Nickel Manganese oxide), etc.) are fascinating materials due to having good optical, electrical, and magnetic properties. The applications of these materials (e.g. gas sensors, catalysts, thermoelectric material, electrode materials in solid oxide fuel cells, etc.) lead to a wide range of interest in their synthesis and structural analysis [8,9,10]. Double perovskite La2CoMnO6 has received enormous research interest because of its reasonably large magneto-capacitance and magneto-resistance. Counting on the method of synthesis, optimum performance, and effective properties of well-defined microstructures are suitable [11]. The Spectroscopic studies [12] on LaMnCoO3 compounds indicate that a mixture of Mn3+ and Mn4+ is present in this series, and therefore a double-exchange mechanism is required to account for the ferromagnetism of these compounds. Normally, in a disordered double perovskite series, each of the B-site cations is in the 3+ oxidation state. However, in the ordered phase, the ferromagnetic behavior appears because of the super interchange interaction between the cations Co2+ and Mn4+ [13, 14]. Therefore, the debate subsists around the electronic state of the transition metals (TMs) in this family of compounds.
Numerous techniques have been advanced to focus on the problem of perovskite purity; the most frequently used of these is the sol–gel citrate (SGC) method. The complexing agent used in SGC is citric acid. This method prepares it possible to obtain a high surface area, but it has the weakness of rather rapid sintering depending on the temperature [15]. Nevertheless, our novel method presented good advantages such as lower temperature compared to the solid-state reactions, better homogeneity, improved reactivity, and new compositions, and better control of stoichiometry, particle size, and purity. Consequently, this novel method has opened new directions for molecular architecture in the synthesis of perovskites.
This paper aims to novel synthesized by using gamma irradiation to obtain a gel product instead of heat treatment for LCMO multifunctional materials. I am reporting in this current article for the first time that, the synthesis of LaCo0.2Mn0.8O3and La2CoMnO6 perovskite materials by modified sol–gel route (citrate-gel method); Gelation step was carried out by gamma radiation in the Cobalt-60 gamma cell source (made in Russia) installed at the National Centre for Radiation Research and Technology, Cairo, Egypt (dose rate 1.2 Gy/h). This method of preparation is environmentally friendly, low cost, high stability, and low toxicity over large-scale commercial applications. I am also describing their electric, dielectric, and magnetic properties to compare with the difference between perovskite and double perovskite materials in properties.
2 Experimental technique
2.1 Materials
LaCo0.2Mn0.8O3 and La2CoMnO6 nanoparticles were synthesized by a modified sol–gel route (citrate-gel method) from their nitrate precursors. Lanthanum Nitrate, Cobalt Nitrate, Manganese (II) acetate tetrahydrate, Citric Acid acts as fuel to speed the reaction, and Nitric Acid was taken up from Alfa Aesar with purity (99.99%). Gelation by gamma radiation was done in the Cobalt-60 gamma cell source (made in Russia), nominated at the National Centre for Radiation Research and Technology, Cairo, Egypt (dose rate 1.33KGy/h).
2.2 Synthesis procedure
Appropriate quantities of these components were dissolved in a minimum amount of water. This solution was mixed with the citric acid solution in a 1:1 volume ratio. A small quantity of nitric acid (1/5 the quantity of water) was also added to convert the corresponding precursors into their respective nitrates. The obtained solution was stirred at 70 °C and then the prepared solution was entered into the gamma irradiation cell at 25 kGy until it reached a gel-like consistency. The obtained gel was dried in an oven; the temperature was raised slowly to 250 °C and preserved overnight to produce a solid amorphous citrate precursor. The obtained powder was crushed, sieved, and calcined at 900 °C in the air for 6 h.
2.3 Characterizations
2.3.1 Structural and morphology
X-ray diffraction (XRD) patterns were acquired by utilizing XRD-6000 Shimadzu. X-ray diffractometer used CuKα radiation (λ = 1.54059 Å) in the range 2θ between (4 and 90°). The TGA analysis was executed using a Shimadzu analyzer model TGA- 50 in the N2 atmosphere at a heating rate of 10 °C/min up to 1000 °C. The morphological structure of the samples was deliberated using scanning electron microscopy (SEM) model JEOL-JSM5400, with an acceleration voltage of 30 kV. High-resolution transmission electron microscopy (HRTEM) images were acquired on a JEM-2010 microscope (Jeol, Akishima, Japan) (with an accelerating voltage of 200 kV and a resolution of 0.14 nm). The images were examined and filtered using the Digital Micrograph program (GATAN, Pleasanton, CA, USA). A suspension of perovskite particles in ethanol was deposited onto a copper grid support with an ultrasonic disperser. Fourier transform infrared spectroscopy (FTIR), BRUKER Vertex 70 optics layout, was used to depict the molecular structure and chemical bonds of LaCo0.2Mn0.8O3 and La2CoMnO6.
2.3.2 Vibrational and intrinsic dielectric measurements
Raman scattering spectra were taken between 4 and 1200 cm−1 at room temperature in a pseudo-back scattering configuration. Jobin–Yvon model U1000 double monochromator was utilized. The laser light source from a Spectra-Physics 2020 argon-ion laser is 514.5 nm line radiations. Each Raman scattering spectrum is the average of 12 consecutive scans acquired at a spectral resolution of 2 cm−1. To realize the frequency stability and the accuracy of the apparatus, the Raman spectrum of silicon was recorded. Raman scattering spectra were recorded using a low excitation power of 10 mW, to avert sample photodecomposition or denaturation.
The 2 point-probe method was utilized to measure DC conductivity using a Keithley model 6517 A. The dielectric characterization dependence on frequency was done using an LCR meter (model 3532, HIOKI, Ueda, Nagano, Japan), with a capacitance measurement ~0.0001 pF and in a frequency region (50 Hz and 5 MHz).
2.3.3 Magnetic and ESR spectra measurements
The Vibrating Sample Magnetometer device Lake Shore 7410 was employed to check the magnetic properties. Magnetization hysteresis loop was collected at 300 K and up to 20 kOe. Temperature sweeps were collected between 100 and 300 K. ESR signal was measured at room temperature by an X-band EMX spectrometer (Bruker, Germany) using a standard rectangular cavity of ER 4102 to record the free radicals created in exciting material by the magnetic field. The operating parameters applied during the ESR experiment are microwave power (mW); modulation amplitude (Gauss); modulation frequency (at 100 kHz); sweep width (Gauss); microwave frequency (GHz); time constant (ms); conversion time (ms) and sweep time (s).
3 Results and discussions
3.1 Structure and morphology studies
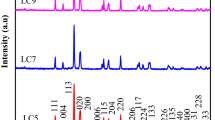
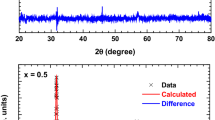
Room-temperature XRD measurements were executed over a 2θ range of 10–90° using a Cu-Kα source, as demonstrated in Fig. 1a for LaCo0.2Mn0.8O3 and La2CoMnO6 samples. XRD patterns showed that the samples are quite crystalline without having any impurity phases. In additional meaning, have not extra reflection peaks than those of pure perovskite phase are detected. The crystal structure approved by La2CoMnO6 compound depends on cationic size, charge, and the ionic radii of rare earth, transition metal, and oxygen ratio, which are computed by tolerance factor [16]. With Matching to the tolerance factor, components of the double perovskite category have numerous structures, for instance: (i) cubic for 1.05 > t/ > 1, (ii) tetragonal for 1.00 > t/ > 0.97, and (iii) monoclinic/orthorhombic for t/ < 0.9. The two prepared samples were indexed according to the orthorhombic structure lattice that belongs to the space group Pbnm, in agreement with ICSD no. 00-072-1186 by using the software “POWD MULT”. Figure 1b the x–y date and print-screen from image “Full-Prof program” for La2CoMnO6 Double Perovskite sample. Also, we can calculate the crystallite size (D) and the microstrain (ε) of the samples using the Williamson-Hall (WH) analysis [17], in which these parameters are calculated using the following relation:
where λ is the wavelength of incident radiation, k a dimensionless shape factor which assumes a typical value of ~0.9, and β the full width at half maximum (FWHM) of the diffraction peaks. Figure 2 represents β cos θ/λ vs. sin θ/λ plot for the checked samples. The amount of strain is given by the slope of the straight line. While the crystallite size is given by the intercept on the β cos θ/λ axis. Table 1 gives the obtained crystallite sizes (D) and microstrain (ε) values. The D values declare that the studied samples are nanometric. The considerable straight lines in the WH plotting signalize no dispersion in particle size and microstrain thus indicating that the samples display homogeneous particle size distributions and microstrain [18].
The morphology of the surface and particle distributions of the prepared samples was examined by SEM. The obtained photographs can be found in Fig. 3. From which it is obvious that the samples under study composed of multiple irregular flowers structure distributed in the less crystalline matrix. The photographs affirm that the particle aggregation became bigger and uniformly distributed for La2CoMnO6 sample. This signifies an increase in the crystalline character, and that sample is highly dense which confirms XRD results.
High-resolution TEM (HRTEM) can be used to demonstrate different morphologies and properties of different perovskite particles [19, 20]. The HRTEM images clearly showed orthorhombic phase with high crystallinity in the case of LaCo0.2Mn0.8O3, while HRTEM La2CoMnO6 showed hexagonal distorted rhombohedral phases. The phase purity of the as-prepared samples shows in Fig. 4; it shows that the samples mainly consist of well-crystallized, and no zones of amorphous phases were being found. The particle size of LaCo0.2Mn0.8O3 is substantially smaller than in the case of La2CoMnO6. A full agreement between the particle sizes was investigated by HRTEM and the XRD data was observed.
3.2 Thermal decomposition
Figure 5 shows TGA and DTG thermograms of the synthesized LCMO nanoparticles. The figure illustrates that the decomposition process in the temperature extent from R.T. up to 1000 oC occurred in three weight loss regions. Thermal analysis can be used to determine thermal stability and decomposition prepared perovskite temperature. The optimum calcination temperature for perovskite can be determined using thermal analysis (TGA and DTG) of the citrate complex of (La, Co, and Mn) [19, 21, 22]. The TGA curve shows a weight loss of ~13, and 10% for LaCo0.2Mn0.8O3, and La2CoMnO6 respectively, which due to the release of moisture absorbed in the sample. A Collapse (concurrent large weight loss) of the citrate complex could be ~45%, and 28% for LaCo0.2Mn0.8O3, and La2CoMnO6 respectively, which due to the reaction of nitrates metals with citric acid has been occurred at a temperature of about 330 °C with a smooth weight loss step and sharp exothermic peak. La2CoMnO6 formation was achieved at ~590 °C through a small weight loss step [21] ~17%, and 23% for LaCo0.2Mn0.8O3, and La2CoMnO6 respectively. Otherwise, there is no change in weight or enthalpy which occurs in the perovskite samples at temperatures as high as 800 °C. Also, the combustion is completed and no organic matter is present in the perovskite samples. So, there is no hard evidence phase transition occurs in the perovskite samples above 800 °C. Moreover, the completeness of nitrate → oxide thermal conversion with the help of a thermobalance with high resolution occurred up to 550 °C, which is performed in a closed furnace that controls the temperature and often allows inert gases N2.
Due to the depletion of citric acid in the perovskite samples and the observed evolving gas (NO2 + CO2) during the second heat step. Thus, to explain both the apparent thermal characteristic of the second process and the observed emission of nitrogen dioxide, it is thought that the general reaction following the production of LaCo0.2Mn0.8O3 occurs:
Note that from the above equation, there is no well-developed molecular oxygen, the determined ratio of citric acid/nitrate in the mixed compounds was 0.04, less than the theoretical value (0.27) computed for stoichiometric. Accordingly, the heat emission here is less than the expected stoichiometric reaction and thus limits completion of the direct reaction to perovskites materials.
3.3 Characteristics of bonding by FTIR
FTIR data for as-prepared samples are displayed in Fig. 6. The phase composition and purity of the prepared samples were described using the FTIR spectrum. We get the characteristic bonding, its vibration, and intensity from the amplitude of its absorbance. Normally, the main absorption band of the metal oxide bonds in the perovskite is in the range around 500–600 cm−1, which the internal motion of a charge in Mn–O–Mn bond length in MnO6 octahedral [23].
In LaCo0.2Mn0.8O3, the concentrated absorption band observed at 577 cm–1 can be appointed to Mn–O stretching vibrations formed by the octahedral MnO6 group. In La2CoMnO6, the band due to distorted stretching vibrations of −CH2 groups can be observed at 2923 cm−1 [24]. The absorption peaks around 1059 cm–1 stands for the presence of carbonate [25]. The band 719 cm−1 is due to C−H bending [26]. The bands around 589.5 are distinctive metal–oxygen bond internal phonon modes of MnO6 octahedral. The stretching mode is associated with the change of Mn–O–Mn bond length and the bending mode incorporates the change of Mn–O–Mn bond angle. The feature of the stretching and bending modes at transmission spectra demonstrates that the perovskite structure of LCMO has been formed, which is convenient with the result of XRD.
3.4 Raman spectroscopy
To know the local structure of different micro-phases or macro-phases presented in many manganites [26, 27], we can use Raman Spectroscopy. Figure 7 shows the room-temperature Raman spectra of as-prepared samples. It has been found that the spectra are essentially dominated by two broad peaks at 275.687 and 644.012 cm−1 for LaCo0.2Mn0.8O3 sample, and 271.64 and 667.634 cm−1 for La2CoMnO6 sample, which can be appointed to antisymmetric stretching mode (or Jahn–Teller stretching mode, Ag) and symmetric stretching mode (Bg) of the (Co/Mn) O6 octahedra, respectively, and are mightily related to lattice distortions, as well as the magnetic properties [28].
Moreover, we mention that the intensity of 644.012 cm−1 mode is higher and sharper in LaCo0.2Mn0.8O3 sample than the intensity of 667.634 cm−1 in La2CoMnO6 sample; this is corresponding to X-ray data, where there is an increase in strain and distortion in the lattice for La2CoMnO6. It is appreciated that for the La2CoMnO6 compound, the lattice is structurally distorted in two ways: the MnO6 and CoO6 octahedral present a strong Jahn–Teller cooperative deformity due to Mn3+ and Co3+ ions, and the octahedral is tilted to optimize the La–O bond lengths. The maintenance of nonstoichiometric, including non-Jahn–Teller ions and Jahn–Teller ions, which can resemble the cation, associated defects, or oxygen, participated in the lattice distortions [29]. As declared overhead, the entity of oxygen vacancies encouraged Mn3+ and Co3+ Jahn–Teller ions which increase the lattice distortion performing in the broadening of the Raman peaks and the decrease of peak intensity. The shifting of the position of the peak in two samples may take place, due to the surface strain of the crystal structure [30]. There are extra Raman modes at 852.403 cm−1 for LaCo0.2Mn0.8O3 sample, and 876.422 cm−1 for the La2CoMnO6 sample which may be due to some distortion in the lattice or associated with stretching overtones. Table 2 shows the values of Peaks Position, FWHM, Height, and Area.
3.5 Magnetic properties
Figure 8 illustrates the magnetization data of the prepared nanoparticle samples at room temperature. The figure depicts a clear hysteresis with low coercivity and saturation magnetization at around 20 kOe. It is practically like a paramagnetic. At the very low field, it displays negligible loop like behavior, due to the spin exchanges. The moment (emu/g) resembles the previously reported values [31, 32]. The evidence of the inherent phenomenon is based on the careful analysis of the magnetism on the surface of the nano-structured magnetic grains.
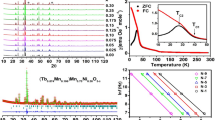
The alteration of magnetization with the temperature for LCMO samples in the temperature extent 100–300 K at an applied field of 5 kOe is demonstrated in Fig. 9. The figure declares that the magnetization of field cooling (Fc) decreases as temperature increases. The obtained transition temperatures are shown in Table 3 for the as-prepared samples. The figure shows a clear single ferromagnetic (FM) transition temperature around TC ≈ 220 K° for LaCo0.2Mn0.8O3 and TC ≈ 229 K° for La2CoMno6 samples. These achieved results declare that the magnetic phase transition temperature gradually broadens and shifts to lower temperatures as the grain size decreases. This indicates that the magnetic surface effect occurs in these grainy samples. Initial studies reveal that coupling among magnetic ions at the surfaces is much lower than that in the cores as a result of a massive number of hanging bonds and non-coordinating of atoms existing at the surface [33, 34].
In the prepared La2CoMnO6 nanoparticle sample, the distinctive magnetic transitions at different temperatures were remarked by the presence of various magnetic domains with different categories of exchange interactions, like Co3+–O–Mn3+ and Co2+–O–Mn4+ super-exchanges. In Co2+–O–Mn4+ super-exchanges in abundantly ordered, are dependable for higher ferromagnetic ordering temperature (Tc ~229 K°) [35], while Co3+–O–Mn3+ super-exchange interaction is responsible for the transition temperature [36]. Therefore, the variation of oxidations states of transition metal ions acts a vital role in their magnetic properties. It suggests that due to the substitution, the manifestation of the spin-glass state is suppressed. This fact is beyond reinforced by the ESR (electron spin resonance spectroscopy) will be studied.
Figure 10 shows the temperature reliance of magnetic susceptibility data of LCMO samples in the region (100–300 K). The decline was noticed in the 1/χ (T) curves and is explained by Griffiths phase (GP) [37, 38], which was initially recommended for randomly diluted Ising ferromagnets. This tendency of 1/χ (T) follows the general features of a GP phase where inverse susceptibility deviates from Curie–Weiss (CW) explanation at T → Tc. The Curie–Weiss law is specified by
with Cm being the Curie–Weiss constant and θ the Curie–Weiss temperature.
For all samples, the positive value of θ = Tc confirms a dominant FM interaction. The effective moment μeff is linked to the Curie constant Cm by the equation Cm = (μeff)2/2KB where KB is the Boltzman constant, hence it is probable to acquire it for the studied samples (Table 3).
3.6 ESR spectra
The electron spin resonance method is used for studying unpaired electrons or investigating oxygen-vacancy-related defects in materials. In ESR, electron spins are excited, but not the spins of atomic nuclei. Since the spin of the electron interacts with the magnetic field and spin is not been zero. Electron spin resonance (ESR) with frequency sweep and field sweep modes was used to record the coplanar waveguide broadband spectrometer. Thus, we can obtain the spectroscopic splitting factor (g) to the material. ESR lines involve a superposition of two resonance modes, i.e., ferromagnetic and paramagnetic. Figure 11 shows the ESR spectra for LCMO nanoparticle samples. It has been found that the ferromagnetic resonance line has been seen in a smaller magnetic field. The ferromagnetic contribution may have occurred from the clusters of ferromagnetic ions in the samples. The larger linewidth is observed, due to the smaller degree of exchange restricting that can be expressed by Jahn–Teller ion behavior. Jahn–Teller of cobalt ions (Co+3, Co+2) were irregular distribution in the sample, becoming it hard to identify a pattern. In LaCo0.2Mn0.8O3 the g-factor values g‖ ~1.9047 and g┴ ~1.997, while in La2CoMno6 only for this orbital 3d electron g‖ ~2.00. ESR measurement will hint at the occurrence of Jahn–Teller glass comparable to the spin-glass behavior in the LCMO system, which has been ascribed as the semiconductor-to-metal transition. The role of La magnetism in La2CoMno6 is not noteworthy because of Co–Mn sublattice interacts weakly with the rare earth in double perovskites [39].
3.7 Dielectric measurements
The dielectric constant was obtained using the equation:
Where C is the capacitance, d is the thickness, ɛ0 is the permittivity of free space and A is the cross-section area of the pellet. Figure 12 shows the dielectric constant and loss dependence on frequency for LCMO samples. The dielectric constant decreases with increasing frequency, analogous to many of the dielectric materials.
At low frequency, the dielectric constant has a very high value because of the presence of numerous types of polarization (i.e. ionic, dipolar or orientation, electronic and space charge). While at high frequency the dielectric constant was described by the main features of the polar dielectrics. The value of the dielectric constant will be small and polarons cannot be able to pursue the electric field at a higher frequency, which results in frequency-independent polarization [40,41,42,43,44].
At low frequency, the value of the dielectric loss is high because of the migration of ions in the material. But, at high-frequency, ion vibrations perhaps the only source of dielectric loss, and hence it has the minimum value. The distinctiveness of low dielectric constant and dielectric loss with frequency suggests that the samples possess reinforced optical quality with lesser defects and this parameter is of indispensable importance for various nonlinear optical materials and their applications [45]. As well-known as dielectric loss intends the extent of energy used by the applied field for the alignment of dipoles. So, the grains are particularly conducting as compared to the grain boundaries. In other words, at low-frequency, only the passage of charge carriers to the grain boundary produces a huge amount of energy loss.
According to Knoop’s theory [46], the structure is implicated to consist of well-conducting grains separating by poorly conducting grain boundaries. The bulk and grain boundary assist in the total conductivity can be separated by using impedance measurement.
The high dielectric constant and relaxation property in LCMO prepared system was due to the local polarization from Mn4+ and Co2+ and extrinsic Maxwell–Wagner (M–W) polarization was advised to report for its irregular dielectric behavior [47]. On the other hand, the existence of internal boundary layers or external depletion layers creation at the interface of the sample/electrode was due to the Schottky barrier effect. For practical applications, the dielectric tunable materials have an essential high dielectric constant. So, the prepared LCMO nanoparticle system has gained a noteworthy interest because of potential applications of this type of electric tunable devices such as capacitors, phase shifters, microwave communication devices, filters, and oscillators [48, 49].
The impedance measurement as a function of frequency supplies the information about the real resistive part (Z′) and imaginary or reactive part (Z″) of complex impedance. Figure 13 illustrates the variation of Z′ as a function of frequency at R.T. This figure illustrates that the value of Z′ has a high value at low frequency and decreasing with increasing frequency. The obtained Z′ values of LaCo0.2Mn0.8O3 are found to be much smaller than those of La2CoMnO6.
The complex electric modulus (M*) of the material is used to study the bulk, grain boundary, electrode polarization, electrical conductivity, and relaxation process. The other benefit of a complex electric modulus is to overcome the electrode effect. Figure 14 shows that the plots of Z″ and M// against frequency and displays a broad Debye peak. So, the complex electric modulus M* can be deemed using the following relation
The plot of imaginary electric modulus M// and impedance Z″ as a function of frequency is useful to reveal the effect of the smallest capacitance, the largest resistance and to recognize whether a relaxation process is due to short or long-range motion of charge carriers as proposed by Sinclair and West [50]. They reported that if the peaks in M// and Z″ against frequency occurred at the same frequency, the process is long-range and if the peaks occurred at different frequencies then the process is localized. In Fig. 13, we observed that with the increment of the frequency, the value of M// rises. Noticeably, we can be expected that at a frequency above 1 MHz the peak will shift to the right (high-frequency region), which is representative of the relaxation mechanism. The relaxation time is determined by the relation t (M//) = 1/(2π f (M//)), where f (M//) is the frequency at which M// (ω) is maximum. The obtained values of t are 1.22 and 5.31 msec for LaCo0.2Mn0.8O3 and La2CoMnO6, respectively.
The real (Z′) and imaginary (Z″) component of the impedance are plotted as Nyquist or Cole–Cole plot. Hence, various relaxing components such as grain, grain boundary, interface (due to space charge), and the electrode effect are explained by low-frequency, high-frequency, and intermediate-frequency-independent semicircles occurring sequentially. Each relaxing component displays a semicircle whose radius represents the resistance of the individual component. Figure 15 shows the Nyquist plot of LCMO samples at R.T. This figure shows a single semicircle, which occurs on account of a parallel combination of grain resistance (Rg) and grain capacitance (Cg). The apparition of a single semicircle means that the electrical process follows a single relaxation mechanism [51]. An increase in the radius of the semicircle is the representation of the increase in the resistance of LaCo0.2Mn0.8O3 sample [52]. Figure 16 depicts the relation between real and imaginary electric modulus M/ and M// for LaCo0.2Mn0.8O3 and La2CoMnO6 and equivalent circuit. An identical circuit of a series combination of resistances (R) and capacitances (C) is operated for an individual arc. A vanishing of an ideal Debye type of relaxation can be clarified by the CPE (constant phase element) which can be connected to the parallel RC system in the recommended circuit [53].
4 Conclusions
This study has reported the synthesis of LaCo0.2Mn0.8O3 perovskites, and La2CoMnO6 double perovskites by the modified sol–gel route (citrate-gel method) with citric acid as the combustion agent. We have outright a detailed analysis of the action of cation nature in position B on the structural and magnetic characterization of the double perovskite. FTIR spectra and XRD patterns approved the perovskites phase formation for the samples under investigation. The investigated compounds are ferromagnetic and magnetic properties are indirectly affected by the distortion degree of the lattice and disorder on the B/B’ positions. Further, this article has been studied the magnetic properties at low temperature from liquid nitrogen temperature to room temperature. These results suggest that these materials were orthorhombic structure lattice that belongs to the space group Pbnm (62), also there is a divergence between ZFC and FC in magnetization curves. Moreover, these materials have a high Tc single magnetic transition with a strong ferromagnetic (FM) super-exchange interaction between high-spin cations Co2+ and Mn4+. The potential applications of these multifunctional materials were concluded conformist substances in solar cells, electric tunable devices, biomolecular, and gas sensing technologies. Long-range and Debye dielectric relaxation are imputed by electric modulus. The complex impedance spectra show the contribution of grains (bulk) only endorsed by the classical Cole–Cole plot. Especially in this article, the high dielectric tunability of these multifunctional materials has depicted an extraordinary concern of electric devices in these capacitors, phase shifters, microwave communication devices, filters, and oscillators.
Abbreviations
- DP:
-
(Double Perovskite)
- LCMO:
-
(Lanthanum Cobalt Manganese oxide)
- LNMO:
-
(Lanthanum Nickel Manganese oxide)
- FM:
-
(Ferromagnetic)
- TMs:
-
(transition metals)
- DC:
-
conductivity (Direct Current conductivity)
- FC:
-
(Field Cooling)
- ZFC:
-
(Zero Field Cooling)
- GP:
-
(Griffiths Phase)
- TC:
-
(magnetic transition temperature or Curie Temperature)
References
Gheorghiu F, Curecheriu L, Lisiecki I, Beaunier P, Feraru S, Palamaru MN, Musteata V, Lupu N, Mitoseriu L (2015) “Functional properties of Sm2NiMnO6 multiferroic ceramics prepared by spark plasma sintering”. J Alloy Compd 649:151–158. https://doi.org/10.1016/j.jallcom.2015.07.136
Ramesh R, Spaldin NA (2007) Multiferroics: progress and prospects in thin films. Nat Mater 6:21–29. https://doi.org/10.1038/nmat1805
W Prellier, MP.Singh, P.Murugavel, the single-phase multiferroic oxides: from bulk to thin film, 2005, J. Phys. Condens. Matter. 17 R803 https://doi.org/10.1088/0953-8984/17/48/C01
Rogado NS, Li J, Sleight AW, Subramanian MA (2005) Magnetocapacitance and magnetoresistance near room temperature in a ferromagnetic semiconductor: La2NiMnO6, Adv. Mater 17:2225–2227. https://doi.org/10.1002/adma.200500737
Tiwari RM, Gadhvi M, Nag A, Vasanthacharya NY, Gopalakrishnan J (2010) Manganese-mediated ferromagnetism in La2Fe1-xMn2xCr1-xO6 perovskite oxides. J Chem Sci 122:529–538. https://doi.org/10.1007/s12039-010-0087-7
Dimitrovska-Lazova S, Aleksovska S, Tzvetkov P (2015) Synthesis and crystal structure determination of YCo1-xFexO3 (x = 0, 0.33, 0.5, 0.67 and 1) perovskites. J Chem Sci 127:1173–1181. https://doi.org/10.1007/s12039-015-0878-y
Dass RI, B J (2003) Goodenough, Multiple magnetic phases of La2CoMnO6-δ (0 ≤ δ ≥ .05). Phys Rev B 67:014401. https://doi.org/10.1103/PhysRevB.67.014401
Feng C, Ruan S, Li J, Zou B, Luo J, Chen W, Dong W, Wu F (2011) Ethanol sensing properties of LaCoxFe1−xO3 nanoparticles: effects of calcination temperature, Co-doping, and carbon nanotube-treatment. Sens Actuators B Chem 155:232–238. https://doi.org/10.1016/j.snb.2010.11.053
Bin F, Song C, Lv G, Song J, Gong C, Huang Q (2011) La1–xKxCoO3 and LaCo1–yFeyO3 perovskite oxides: preparation, characterization, and catalytic performance in the simultaneous removal of NOx and diesel soot. Ind Eng Chem Res 50:6660–6667. https://doi.org/10.1021/ie200196r
Vulchev V, Vassilev L, Harizanova S, Khristov M, Zhecheva E, Stoyanova R (2012) Improving of the thermoelectric efficiency of LaCoO3 by double substitution with nickel and iron. J Phys Chem C 116:13507–13515. https://doi.org/10.1021/jp3021408
Popa M, Hong LVA, Kakihana M (2003) Nanopowders of LaMeO3 perovskites obtained by a solution-based ceramic processing technique. Phys B Condens Matter 327:233–236. https://doi.org/10.1016/S0921-4526(02)01736-2
Park JH, Cheong SW, Chen CT (1997) Double-exchange ferromagnetism in La (Mn1-xCox)O3. Phys Rev B55:11072. https://doi.org/10.1103/PhysRevB.55.11072
Iliev MN, Gospodinov MM, Singh MP, Meen J, Truong KD, Fournier P, Jandl S (2009) Growth, magnetic properties, and Raman scattering of La2NiMnO6 single crystals. J Appl Phys 106:023515. https://doi.org/10.1063/1.3176945
Singh MP, Truong KD, Jandl S, Fournier P (2010) Multiferroic double perovskites: opportunities, issues, and challenges. J Appl Phys 107:09D917. https://doi.org/10.1063/1.3362922
Merino NA, Barbero BP, Ruiz P, Cadús LE (2006) Synthesis, characterization, catalytic activity and structural stability of LaCo1−yFeyO3±λ perovskite catalysts for combustion of ethanol and propane. J Catal 240(2):245–257. https://doi.org/10.1016/j.jcat.2006.03.020
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32:751–767. https://doi.org/10.1107/S0567739476001551
Williamson GK, Hall WH (1953) X-ray line broadening from filed aluminum and wolfram. Acta Metall 1:22–33. https://doi.org/10.1016/0001-6160(53)90006-6
Gonçalves NS, Carvalho JA, Lima ZM, Sasaki JM (2012) Size–strain study of NiO nanoparticles by X-ray powder diffraction line broadening. Mater Lett 72:36–38. https://doi.org/10.1016/j.matlet.2011.12.046
Vijayakumar C, Kumar HP, Solomon S, Thomas JK, Warriar PRS, Koshy J (2008) Synthesis characterization, sintering and dielectric properties of nanostructured perovskite-type oxide, Ba2GdSbO6, Bulletin. Mater Sci 31(5):719–722. https://doi.org/10.1007/s12034-008-0113-2
Ali SM, Abd Al-Rahman YM, Galal A (2012) Catalytic activity toward oxygen evolution of LaFeO3 prepared by the microwave-assisted citrate method. J Electrochem Soc 159(9):F600–F605. https://doi.org/10.1149/2.063209jes
Galal A, Atta NF, Darwish SA, Abd El Fatah AA, Ali SM (2010) Electrocatalytic evolution of hydrogen on a novel SrPdO3 perovskite electrode. J Power Sources 195:3806–3809. https://doi.org/10.1016/j.jpowsour.2009.12.091
Ghosh S, Dasgupta S (2010) Synthesis, characterization, and properties of nanocrystalline perovskite cathode materials. Mater Sci—Pol 28(2):427–438. http://cgcri.csircentral.net/id/eprint/740
Gao F, Lewis RA, Wang XL, Dou SX (2002) Far-infrared reflection and transmission of La1−xCaxMnO3. J Alloy Compd 347:314–318. https://doi.org/10.1016/S0925-8388(02)00789-2
Annapoorani S, Parameswaran VR (1999) Studies on Complexes of 1,2-Bis-(8-quinoxy) Ethane with Some Bivalent Metal Ions. Asian J Chem 11:763–766
Boo BH (2011) Infrared, and Raman Spectroscopy of Diphenylsilane. Vibrational Assignment by Hartree-Fock and Density-functional Theory Calculations. J Korean Phys Soc 59:3205–3209. https://doi.org/10.3938/jkps.59.3205
Zhao S, Shi L, Zhou S, Zhao J, Yang H, Guo Y (2009) Size-dependent magnetic properties, and Raman spectra of La2NiMnO6 nanoparticles. J Appl Phys 106:123901. https://doi.org/10.1063/1.3269707
Iliev MN, Guo H, Gupta A (2007) Raman spectroscopy evidence of strong spin-phonon coupling in epitaxial thin films of the double perovskite La2NiMnO6. Appl Phys Lett 90:151914. https://doi.org/10.1063/1.2721142
Liu WJ, Shi L, Zhou S, Zhao J, Y LI, Guo Y (2014) Griffiths phase, spin-phonon coupling, and exchange bias effect in double perovskite Pr2CoMnO6. J Appl Phys 116:193901. https://doi.org/10.1063/1.4902078
Iliev MN, Abrashev MV, Litvinchuk AP, Hadjiev VG, Guo H, Gupta A (2007) Raman spectroscopy of ordered double perovskite La2CoMnO6 thin films. Phys Rev B 75:104118. https://doi.org/10.1103/PhysRevB.75.104118
Iliev MN, Abrashev MV, Popov VN, Hadjiev VG (2003) Role of Jahn-Teller disorder in Raman scattering of mixed-valence manganites. Phys Rev B 67:212301. https://doi.org/10.1103/PhysRevB.67.212301
Guo H, Burgess J, Street S, Gupta A (2006) Growth of epitaxial thin films of the ordered double perovskite La2NiMnO6 on different substrates. Appl Phys Lett 89:022509. https://doi.org/10.1063/1.2221894
Huang HY, Cheong SW, Ong NP, Batlogg B (1996) Spin-polarized intergrain tunneling in La2/3Sr1/3MnO3. Phys Rev Lett 77:2041. https://doi.org/10.1103/PhysRevLett.77.2041
Trajanovic Z, Kwon C, Robson MC, Kim KC, Rajaswari M, Lofland SE, Bhagat SM, ForkR D, Venkatesan R,T (1996) Growth of colossal magnetoresistance thin films on silicon. Appl Phys Lett 69:1005. https://doi.org/10.1063/1.117967
Sattler K, Miihlbach J, Recknagel E (1980) Generation of metal clusters containing from 2 to 500 Atoms. Phys Rev Lett 45:821. https://doi.org/10.1103/PhysRevLett.45.821
Cowen JA, Stolzman B, Averback RSA, Han H (1987) Magnetic phase transitions in nanocrystalline erbium. J Appl Phys 61:3317. https://doi.org/10.1063/1.338894
Dass RI, Goodenough JB (2003) Multiple magnetic phases of La2CoMnO6-δ (0≤δ ≥ 0.05). Phys Rev B 67:014401. https://doi.org/10.1103/PhysRevB.67.014401
Blasco J, García J, Subías G, Sánchez MC (2004) Structure and magnetic properties of LaMn1−xMgxO3 compounds. Phys Rev B 70:094426. https://doi.org/10.1103/PhysRevB.70.094426
Salamon MB, Chun SH (2003) Griffiths singularities and magnetoresistive manganites. Phys Rev B 68:014411. https://doi.org/10.1103/PhysRevB.68.014411
Salamon MB, Lin P, Chun SH (2002) Colossal Magnetoresistance is a Griffiths singularity. Phys Rev Lett 88:197203. https://doi.org/10.1103/PhysRevLett.88.197203
Booth RJ, Fillman R, Whitaker H, Nag A, Tiwari RM, Rama nujachary KV, Gopalakrishnan J, Lofland SE (2009) An investigation of structural, magnetic and dielectric properties of R2NiMnO6 (R = rare earth, Y). Mater Res Bull 44(7):1559–1564. https://doi.org/10.1016/j.materresbull.2009.02.003. ISSN0025-5408
Huang T, Chang Y, Chen G, Chung C, Chang Y (2007) J. Electrochem. Soc 154:244–250
Acharya S, Chakrabarti PK (2010) Some interesting observations on the magnetic and electric properties Al3+ of doped lanthanum orthoferrite (La0.5Al0.5FeO3) Solid State Commun., 150:1234. https://doi.org/10.1016/j.ssc.2010.04.006
Jawahar K, Choudhary RNP (2008) Structural and dielectric properties of LaBi2Fe5O12. Indian J Eng Mater Sci 15:203–206. http://nopr.niscair.res.in/handle/123456789/1974
Haron W, Thaweechai T, Wattanathana W, Laobuthee A, Manaspiya H, Veranitisagul C, Koonsaeng N (2013) Structural characteristics and dielectric properties of La1-xCoxFeO3 and LaFe1-xCoxO3 synthesized via metal-organic complexes. Energy Procedi 34:791–800. https://doi.org/10.1016/j.egypro.2013.06.815
Rajarajan K, Mani G, Potheher IV, Jesudurai JGM, Vimalan M, Christy D, Madhavan J, Sagayarai P (2007) Growth, optical, dielectric and ESR studies on tetrathiourea mercury(II) tetrathiocyanato manganate(II): an organometallic complex NLO crystal. J Phys Chem Solids 68:2370–2375. https://doi.org/10.1016/j.jpcs.2007.07.036
Koops CG (1951) On the dispersion of resistivity and dielectric constant of some semiconductors at audio frequencies. Phys Rev 121:83. https://doi.org/10.1103/PhysRev.83.121
Chandrasekhar KD, DAS AK, Mitra C, Venimadhav A (2012) The extrinsic origin of the magnetodielectric effect in the double perovskite La2NiMnO6. J Phys Condens Matter 24:495901. https://doi.org/10.1088/0953-8984/24/49/495901
Kleemann W, Dec J, Wang R, Itoh M (2003) Nonlinear susceptibility, and phase transition in SrTi18O3. Phys Rev B 67:092107. https://doi.org/10.1103/PhysRevB.67.092107
Liang X, Wu W, Meng Z (2003) Dielectric, and tunable characteristics of barium strontium titanate modified with Al2O3 addition. Mater Sci Eng B 99:366–369. https://doi.org/10.1016/S0921-5107(02)00461-0
Shukla A, Choudhary NP, Thakur AK (2009) Thermal, structural and complex impedance analysis of Mn4+ modified BaTiO3 electroceramic. J Phys Chem Solid 70:1401–1407. https://doi.org/10.1016/j.jpcs.2009.08.015
Snclair DC, West AR (1989) Impedance and modulus spectroscopy of semiconducting BaTiO3 showing positive temperature coefficient of resistance. J Appl Phys 66:3850. https://doi.org/10.1063/1.344049
Jyothy PV, ArunKummar KV, Karthiska A, Rajesh R, Unnikrishnan NV (2010) Dielectric and AC conductivity studies of CdSe nanocrystals doped sol-gel silica matrices. J Alloy Comp 493:223–226. https://doi.org/10.1016/j.jallcom.2009.12.060
Sahoo S, Mahapatra PK, Choudhary RNP (2016) “The structural, electrical and magnetoelectric properties of soft chemically-synthesized SmFeO3 ceramics”. J Phys D Appl Phys 49:035302. https://doi.org/10.1088/0022-3727/49/3/035302
Acknowledgements
The authors gratefully acknowledge the generous financial support of Egyptian Atomic Energy Authority (EAEA). This research was supported by National Centre for Radiation Research and Technology (NCRRT). A great thankful for Dr. Mostafa Saied Shalaby (M.S. Shalaby) in Solid-State and Accelerators Department, National Center for Radiation Research & Technology(NCRRT), Egyptian Atomic Energy Authority(EAEA).
Author information
Authors and Affiliations
Contributions
N.M. Yousif: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; visualization; validation; roles/writing—original draft; and review and editing. N. Makram: funding acquisition; resources; software; validation; writing—review and editing. L.A. Wahab: supervision.
Corresponding author
Ethics declarations
Conflict of interest
We the undersigned declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, concerning intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property. We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). I am responsible for communicating with the other authors about progress, submissions of revisions, and final approval of proofs.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yousif, N.M., Makram, N. & Wahab, L.A. Structural, dielectric, and magnetic properties of LaCo0.2Mn0.8O3 and La2CoMnO6 perovskite materials. J Sol-Gel Sci Technol 98, 238–251 (2021). https://doi.org/10.1007/s10971-021-05502-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05502-4