Abstract
CeO2–Nd2O3 microspheres were successfully prepared by internal gelation process using M(OH)m and [MCit∙xH2O] (M = Ce3+, Ce4+, and Nd3+, Cit is (C6O7H5)3−) as precursors. The effects of Nd(NO3)3 content on the stability of precursor solution and on the microstructure of the sintered microspheres were investigated. The gelled microspheres and sintered composite microspheres were characterized by Fourier transform-infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), and X-ray fluorescence (XRF) spectroscopy. The distribution of Nd3+ in the microspheres was also investigated by line scanning of SEM. The results indicated that the citrate salt in the gelled microspheres was [MCit∙xH2O] (M = Ce3+ and Nd3+). Compared with composite microspheres prepared with M(OH)m (M = Ce4+ and Nd3+) as a precursor, the mass fraction of Nd2O3 in composite microspheres prepared with [MCit∙xH2O] as a precursor highly coincided with the theoretical value. There was no concentration gradient in the microspheres and the distribution of Nd3+ was homogeneous. Phase composition of the composite microspheres was Ce0.75−xNd0.25+xO1.85 and CeO2.

The gelation process of microspheres prepared with hydroxide.
Highlights
-
CeO2–Nd2O3 microspheres were prepared by internal gelation process with M(OH)m and [MCit∙xH2O] as precursors.
-
The mass fraction of Nd2O3 in composite microspheres prepared with [MCit∙xH2O] highly coincides with the theoretical value.
-
No concentration gradient existed in the microspheres and the distribution of Nd3+ was homogeneous.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The subcritical accelerator-driven system is considered as an effective method for transmutation of Pu, minor actinides (MAs), and long-lived fission products (LLFPs), which have high radioactivity and lasting radiotoxicity [1,2,3,4]. For avoiding the formation of new transuranium actinides due to the neutron capture by 238U and achieving the maximum transmutation efficiency, inert matrix fuels (IMF) with a ceramic matrix, such as Mo, MgO, ZrO2, and ZrN [4,5,6] are advantageous [5, 7]. Due to structural stability under irradiation, inertia of neutrons, similar sintering behavior, and thermal properties of PuO2 and CeO2 can also be used as an inert matrix material [1, 8,9,10].
Pellet and sphere-pac are two types of IMF. Compared with pellet fuels, sphere-pac fuels have some advantages as the following: the dustless fabrication with sol–gel or gel-casting methods, swelling can be reduced or eliminated by storing gas in the gap or releasing out the gas through the particle boundary [11]. Sphere-pac IMF can be prepared by infiltration of porous ceramic microspheres with MA nitrate solution [7] and co-sol–gel process with nitrate solution containing MA and metal ion to prepare the inert matrix [12]. But the nonuniform porosity of the porous ceramic microspheres, or diffusion process during the thermal treatment, may lead to inhomogeneity of the MA distribution in the microspheres prepared by infiltration technique [12, 13].
As an advanced method that directly converts droplet of precursor solution into microspheres, internal gelation process has been widely used to prepare nuclear fuel and IMF [14,15,16,17,18]. Preparation of CeO2 microspheres has also been investigated by internal gelation, with cerium hydroxide as the precursor [10, 19,20,21,22], and no other precursor was used to prepare CeO2 microspheres. Cerium citrate, as another kind of precursor, which was used to prepare CeO2 powder by sol–gel process [23, 24], can also possibly be used to prepare CeO2 microspheres. Citrate salts were often used to prepare lanthanide oxide [25, 26], and could be used to prepare composite microspheres containing MAs and LLFPs, because lanthanides and trivalent actinides have similar chemical properties.
The aim of this work is to prepare homogeneous CeO2–Nd2O3 microspheres with M(OH)m and citrate salts as precursors by co-sol–gel process. Neodymium ion Nd3+ was used as a surrogate for the trivalent actinides. Due to that the typical IMF contain 5–30 wt% of MA; CeO2–Nd2O3 microspheres with varying amounts of Nd2O3 (5–30 wt%) were prepared. Neodymium ion Nd3+ distribution in the microspheres was investigated.
2 Experimental section
2.1 Preparation of CeO2–Nd2O3 microspheres with M(OH)m (M=Ce4+ and Nd3+) as a precursor
CeO2–Nd2O3 microspheres were synthesized with M(OH)m as the precursor, based on a literature procedure [22]. It was a typical sol–gel process for the preparation of oxide microspheres. Specifically, ammonia (25% concentration, Beijing Chemical Works, China) was dissolved in the 1.6 M Ce(NH4)2(NO3)6 (99% purity, Sinopharm Chemical Reagent Co., Ltd., China) solution, and the pH of the solution could be changed by varying the molar ratio of NH3∙H2O/Ce(NH4)2(NO3)6. Quantitative Nd(NO3)3∙6H2O (99% purity, Shanghai Macklin Biochemical Co., Ltd, China) was dissolved into Ce4+-containing solution to prepare the microspheres with different mass fractions of Nd2O3. Hexamethylenetetramine (HMTA) (99% purity, Sinopharm Chemical Reagent Co., Ltd., China) and urea (99% purity, Sinopharm Chemical Reagent Co., Ltd., China) were dissolved into deionized water to prepare the solution consisting of 3.2 M HMTA and 3.2 M urea. Precursor solution was prepared by mixing the Nd-containing solution and solution containing 3.2 M HMTA and 3.2 M urea with equal volume, then the precursor solution was cooled to 4 °C for at least 30 min to diminish the hydrolysis of HMTA. The precursor solution was dropped into 90 °C silicon oil. The droplets could maintain spherical shape due to their surface tension. On heating by the hot silicon oil, the spherical droplets solidified within a few seconds by the decomposition of HMTA. The gelled microspheres were aged for at least 30 min. Then, the microspheres were washed with trichloroethylene (TCE) (99% purity, Sinopharm Chemical Reagent Co., Ltd., China) to remove the silicone oil on the surface of the gelled microspheres. A concentration of 0.5 M ammonia was used to remove the NH4NO3 and uncreated urea, etc. Then, the gelled microspheres were treated with hydrothermal treatment to decompose the urea resin generated during the internal gelation process. The spheres were washed with distilled water to remove the organic compound and then washed with propylene glycol methyl ether (99% purity, Sinopharm Chemical Reagent Co., Ltd., China) to remove water in the microspheres. Then, the washed microspheres were dried at 60 °C for at least 12 h, and sintered at 1400 °C for 2 h.
2.2 Preparation of CeO2–Nd2O3 microspheres with citrate salts as precursors
Ce(NO3)3∙6H2O (99.5% purity, Sinopharm Chemical Reagent Co., Ltd., China), and anhydrous citric acid (H3Cit) (99.5% purity, Sinopharm Chemical Reagent Co., Ltd., China) were dissolved into deionized water to prepare a solution consisting of 2 M cerium nitrate and 2 M citric acid. Quantitative Nd(NO3)3∙6H2O (99% purity, Shanghai Macklin Biochemical Co., Ltd., China) was dissolved into Ce3+-containing solution to prepare the microspheres with different mass fractions of Nd2O3. HMTA and urea were dissolved into deionized water to prepare the solution consisting of 3.0 M HMTA and 3.0 M urea, hereafter noted as H–U solution. Both solutions were cooled to 4 °C for at least 30 min. Under magnetic stirring, the Nd3+-containing solution and the H–U solution was mixed with equal volume, then the acrylic acid (AA) (Sinopharm Chemical Reagent Co., Ltd., China) was added into the solution, and the molar ratio of AA/HMTA was unity. Then, 0.4 wt% N,N′-methylenebisacrylamide (97% purity, Sinopharm Chemical Reagent Co., Ltd., China) and 0.2 wt% ammonium persulfate (99% purity, Sinopharm Chemical Reagent Co., Ltd., China) were also added into the precursor solution. The final solution was stirred, until a clear solution was obtained, and then cooled to 4 °C for at least 10 min to diminish the hydrolysis of HMTA and restrain the polymerization of AA.
The solution cooled to 4 °C was dropped into silicon oil of 90 °C with a syringe to form gel microspheres. The spheres were aged for 2 h after all of the gelled spheres settled at the bottom of the column to make sure that the urea resin could be modified thoroughly. The gelled spheres were washed successively by using TCE to remove the silicon oil on the surface of the gelled microspheres and propylene glycol methyl ether to remove the TCE. Compared with that in the “Preparation of CeO2–Nd2O3 microspheres with M(OH)m (M=Ce4+ and Nd3+) as a precursor” section, the present washing treatment produced less waste liquid, and fewer steps were needed. The washed spheres were dried for at least 6 h in an oven at 60 °C. Finally, the spheres were sintered in atmospheres at 1400 °C for 2 h.
2.3 Characterization
A digital pH meter was used to measure the pH of the precursor solution before it was dropped into the hot silicon oil. An LVDV-1 digital rotation viscometer was used to measure the viscosity, and the rotation speed of the rotator was 6 rpm. The infrared spectra of the samples were recorded by VERTEX 70 (Burker, Germany). The phase identification of sintered microspheres was done by using D/max-2500 X-ray diffraction (Rigaku, Japan). A Hitachi S-5500 scanning electron microscope (Hitachi, Japan) was used to observe the microstructure of the microspheres and homogeneity of the neodymium element in microspheres. An XRF spectrometer (Thermo Fisher, China) was used to measure the mass fraction of CeO2 and Nd2O3 in the microspheres.
3 Results and discussion
3.1 Characterization of sol precursor containing citrate salts
As previous investigation indicated, the Ce4+ and Nd3+ of the precursor solution would exist in the forms of Ce(OH)4 and Nd(OH)3 in the gelled microspheres prepared by internal gelation process [27, 28]. The citrate salts have two existing forms, namely [MCit∙xH2O] and [M2(HCit)3∙2H2O], where M=Ce3+ and Nd3+, Cit stands for (C6O7H5)3− and HCit for (C6O7H6)2− [23]. The states of citrate salts in the gelled microspheres were characterized by Fourier-transform infrared (FT-IR) spectroscopy and is shown in Fig. 1. Figure 1 displays a typical infrared spectrum of citrate salts, whose characteristic bands are at 1577 cm−1 and 1384 cm−1 [24]. The characteristic bands of HMTA, urea, nitrate, and citric acid disappear, which indicate that HMTA, citric acid,and urea were hydrolyzed completely. The difference of the infrared spectra between [MCit∙xH2O] and [M2(HCit)3∙2H2O] is whether there are four bands in the region 1380–1460 cm−1. The citrate salt with four bands in its infrared spectra in the region is [M2(HCit)3∙2H2O] [23]. It can be concluded that citrate salt in the gelled microspheres was [MCit∙xH2O].
3.2 Preparation of composite microspheres with 30 wt% Nd2O3
Since the typical IMF contain 30 wt% of MA, composite microspheres with 30 wt% Nd2O3 prepared with the M(OH)m (M=Ce4+ and Nd3+) were compared with microspheres prepared with the [MCit∙xH2O] (M=Ce3+ and Nd3+). Microspheres were prepared with the M(OH)m that the NH3∙H2O/Ce4+ molar ratio was 1 and [MCit∙xH2O] as precursors. In order to investigate the difference of the preparation of CeO2–Nd2O3 between M(OH)m and [MCit∙xH2O] microspheres, mass fractions of Nd2O3 in the sintered microspheres were determined by the XRF spectrometer, as shown in Table 1. As can be seen from Table 1, ∆m was the difference of the determined value and theoretical value; the determined value of Nd2O3 in the sintered microspheres prepared by M(OH)m was different from the theoretical value with the difference of −9.52 wt%, which indicates that a large amount of Nd3+ was lost in the aging and washing processes. Figure 2 is the distribution of Nd3+ in a crosssection of the sintered microspheres prepared using M(OH)m and [MCit∙xH2O] as precursors. It was recorded with the line scanning of SEM. As can be seen from Fig. 2a, an obvious concentration gradient existed in the microspheres, and the concentration of Nd3+ at the edge of the microspheres was higher than that in the center. This was presumably attributed to the large difference of the solubility product constant Ksp between Ce(OH)4 (2 × 10−48) and Nd(OH)3 (Ksp = 3.2 × 10−22). The results indicated that the transformation of Ce4+ into Ce(OH)4 and Nd3+ to Nd(OH)3 did not occur simultaneously. Transformation of Ce4+ into Ce(OH)4 started much earlier than that of Nd3+ into Nd(OH)3. During the aging process in the gelled microspheres, water was produced and released out of the microspheres. The neodymium ion Nd3+ that had not been transformed into Nd(OH)3 can be dissolved in the water. When the water was carried out of the microspheres, the Nd3+ ions dissolved in the water were also carried out of the microspheres, which led to the loss of Nd3+ in the gelled microspheres. When the gelled microspheres were washed with 0.5 M NH3∙H2O, whose pH was 11.47, Nd3+ could be transformed into Nd(OH)3 completely. The gelation process of the microspheres prepared with M(OH)m as a precursor is schematically demonstrated in Fig. 3.
However, there was almost no loss of Nd3+ in the microspheres prepared by [MCit∙xH2O], as shown in Table 1. As can be seen in Fig. 2b, no concentration gradient existed in the microspheres, and the distribution of Nd3+ was homogeneous, because of the small difference of formation constants between [CeCit∙xH2O] with the lgK of 9.85 and [NdCit∙xH2O] with the lgK of 9.70. The gelation process of microspheres prepared with [MCit∙xH2O] as a precursor is schematically demonstrated in Fig. 4.
Since the pH has an important effect on the gel formation, the effect of NH3∙H2O/Ce4+ molar ratio on the composite microspheres should also be investigated. Table 2 shows the effect of NH3∙H2O/Ce4+ molar ratio on the composite microspheres prepared with cerium hydroxide as the precursor, with the NH3∙H2O/Ce4+ molar ratio varying from 2 to 3.5. With the increasing of the NH3∙H2O/Ce4+ molar ratio, the mass fraction of Nd2O3 in the sintered microspheres became higher with the differences varying from −9.05 to −3.15 wt%. It indicates that more Nd3+ was retained during the aging and washing processes.
Although the NH3∙H2O/Ce4+ molar ratio varied to 3.5, significant differences between the determined value and the theoretical value of Nd2O3 in the sintered microspheres prepared with M(OH)m as the precursor still existed, as shown in Table 2. The sharp increase of the viscosity of the precursor solution is considered to be an onset of the gelation. As can be seen from Fig. 5, the onset earlier with the NH3∙H2O/Ce4+ molar ratio went from 1 to 3.5. The increase in the NH3∙H2O/Ce4+ molar ratio led to the increase in the mass fraction of Nd2O3 in the sintered microspheres, but the stability of the precursor solution got worse. The stabilization time of the sol had been shortened to 20 min, when the NH3∙H2O/Ce4+ molar ratio was 3.5. The results indicate that the more ammonia was added to the solution, the more effectively hydrolysis processes were eliminated and the faster hydroxide was formed.
3.3 Preparation of CeO2–Nd2O3 microspheres with citrate salts as precursor
As discussed in the “Preparation of composite microspheres with 30 wt% Nd2O3” section, the determined mass fraction of Nd2O3 in composite microspheres prepared by [MCit∙xH2O] highly coincides with the theoretical value. Different quantities of Nd(NO3)3∙6H2O were added into the precursor solution to study whether the addition of Nd3+ affect the stability of precursor solution and the performance of CeO2–Nd2O3 microspheres.
Figure 6 shows the variation in pH (a) and viscosity (b) of the precursor solutions, with different contents of Nd3+. As shown in Fig. 6a, with the increase of Nd3+ in the precursor solution, the initial and ultimate pH of the precursor solution became slightly lower. As shown in Fig. 6b, the onset of gelation became slightly lower with the increase of Nd3+. The results indicate that the stability of precursor solutions did not change much with the increase of Nd3+.
Mass fractions of Nd2O3 in the sintered microspheres prepared by [MCit∙xH2O], with different contents of Nd3+, were measured by XRF spectrometry and the results are presented in Table 3. Very small differences existed between the determined value and the theoretical value, indicating that the determined mass fraction of Nd2O3 in composite microspheres highly coincides with the theoretical value. The results demonstrate that the addition of Nd3+ did not affect the gelation process of [MCit∙xH2O] in the microspheres.
Figure 7 is the surface of the sintered microspheres with different mass fractions of Nd2O3. Figure 7 indicates that the microspheres have good sphericity. Figure 8 is the cross section of the sintered microspheres with different mass fractions of Nd2O3. The cross sections are porous, and are composed of irregularly shaped particles, which is a feature present in the preparation of all lanthanide oxides [24]. All results indicate that the addition of Nd3+ did not affect the microstructure of microspheres.
Figure 9 is the Nd3+ distribution in cross section of the sintered microspheres with different mass fraction of Nd2O3. Figure 9 indicates that, a small undulation exists in the distribution of Nd3+ in the microspheres, which was mainly caused by topographical variation of the cross sections of the sintered microspheres. Without this undulation, the distribution curves were basically a horizontal straight line. The results indicate that no concentration gradient existed in the microspheres, and the distribution of Nd3+ was homogeneous.
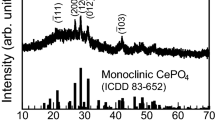
The crystalline structure of the sintered microspheres was determined using XRD. Figure 10 is the XRD patterns of sintered microspheres, with different mass fractions of Nd2O3. As we can see from Fig. 10a, all of the samples show diffraction peaks of Ce0.75−xNd0.25+xO1.85 solid solution (JCPDS Card No. 28-0266) and CeO2 (JCPDS Card No. 81-0792). As shown in Fig. 10b, the deviation of the XRD peak toward the lower angle indicates that Ce4+ ions were progressively replaced by Nd3+ ions, leading to the expansion of the lattice parameter, in accordance with the Vegard’s law. The results indicated that the phase of the composite microspheres was ceria-based solid solution, with the composition of Ce0.75−xNd0.25+xO1.85 and CeO2.
4 Conclusions
CeO2–Nd2O3 microspheres have been prepared by internal gelation process, with M(OH)m and [MCit∙xH2O] (M=Ce3+, Ce4+, and Nd3+, Cit is (C6O7H5)3−) as precursors, respectively. The preparation process of composite microspheres with M(OH)m (M=Ce4+ and Nd3+) led to large losses of Nd3+. However, there was no loss of Nd3+ in the preparation process of composite microspheres with [MCit∙xH2O] (M=Ce3+ and Nd3+) as a precursor. Compared with microspheres prepared with M(OH)m, the mass fraction of Nd2O3 in composite microspheres prepared with [MCit∙xH2O] as precursor highly coincided with the theoretical value. The distribution of Nd3+ in the sintered composite microspheres was homogeneous. The CeO2–Nd2O3 microspheres prepared with citrate salts as precursors were composed of Ce0.75−xNd0.25+xO1.85 and CeO2.
References
Osaka M, Miwa S, Tachi Y (2006) Simple fabrication process for CeO2–MgO composite as surrogate for actinide-containing target for use in nuclear fuel. Ceram Int 32:659–663
Haas D, Fernandez A, Na¨stren C, Staicu D, Somers J, Maschek W, Chen X (2006) Properties of cermet fuels for minor actinides transmutation in ADS. Energy Convers Manag 47:2724–2731
Haas D, Fernandez A, Staicu D, Somers J, Maschek W, Liu P, Chen X (2008) CERMET fuel behavior and properties in ADS reactors. Energy Convers Manag 49:1928–1933
Oigawa H, Tsujimoto K, Nishihara K, Sugawara T, Kurata Y, Takei H, Saito S, Sasa T, Obayashi H (2011) Role of ADS in the back-end of the fuel cycle strategies and associated design activities: the case of Japan. J Nucl Mater 415:229–236
Maschek W, Chen X, Delage F, Fernandez-Carretero A, Haas D, Matzerath Boccaccini CM, Rineiski A, Smith P, Sobolev V, Thetford R, Wallenius J (2007) Accelerator driven systems for transmutation: fuel development, design and safety. Prog Nucl Energy 50:333–340
Guo T, Wang C, Lv JL, Liang TX (2016) Preparation of mesoporous zirconia microspheres as inert matrix. J Nucl Mater 481:66–72
Degueldre C, Paratte JM (1999) Concepts for an inert matrix fuel, an overview. J Nucl Mater 274:1–6
Katalenich JA (2014) Production of monodisperse, crack-free cerium oxide microspheres by internal gelation sol–gel methods. University of Michigan, Ann Arbor, MI, PhD diss.
Ye B, Miao JL, Li JL, Zhao ZC, Chang ZQ, Serra CA (2013) Fabrication of size-controlled CeO2 microparticles by a microfluidic sol–gel process as an analog preparation of ceramic nuclear fuel particles. J Nucl Mater 50:774–780
Katalenich JA, Kitchen BB, Pierson BD (2018) Production of monodisperse cerium oxide microspheres with diameters near 100 μm by internal-gelation sol–gel methods. J Sol–Gel Sci Technol 86:329–342
Wang L, Liang TX (2012) Ceramics for high level radioactive waste solidification. J Adv Ceram 1:194–203
Yang YT, Li X, Fu CF, Song T, Chang ZQ (2015) Fabrication of uniform Ce/Eu oxide microparticles by a microfluidic Co-Sol–Gel process as an analog preparation of MA-bearing ceramic nuclear fuel particles. Nucl Sci Eng 181:216–224
Fernandez A, Haas D, Hiernaut JP, Konings R, Ottmar H, Somers J, Staicu D (2006) Overview of ITU work on inert matrix fuels. In 9th IEMP.
Sood DD (2011) The role sol–gel process for nuclear fuels-an overview. J Sol–Gel Sci Technol 59:404–416
Gao Y, Ma JT, Zhao XY, Hao SC, Deng CS, Liu B (2015) An improved internal gelation process for preparing ZrO2 ceramic microspheres without cooling the precursor solution. J Am Ceram Soc 98:2732–2737
Kumar A, Radhakrishna J, Kumar N, Rajesh VP, Dehadrai JV, Deb AC, Mukerjee SK (2013) Studies on preparation of (U0.47,Pu0.53)O2 microspheres by internal gelation process. J Nucl Mater 434:162–169
Sun X, Ma JT, Chen XT, Li ZQ, Deng CS, Liu B (2018) Sol–gel preparation of ZrC–ZrO2 composite microspheres using fructose as a carbon source. J Sol–Gel Sci Technol 86:431–440
Sun X, Ma JT, Zhao XY, Hao SC, Wang TW, Li ZQ, Deng CS, Liu B (2018) Fabrication process study of UCO composite ceramic microspheres with fructose as a carbon source by internal gelation and carbothermic reduction. J Nucl Mater 511:235–241
Hunt RD, Collins JL, Johnson JA, Cowell BS (2017) Production of 75–150 mm and <75 mm of cerium dioxide microspheres in high yield and throughput using the internal gelation process. Ann Nucl Energy 105:116–120
Katalenich JA (2017) Production of cerium dioxide microspheres by an internal gelation sol–gel method. J Sol–Gel Sci Technol 82:654–663
Li X, Yang YT, Fu CF, Huang QY, Sheng LS, Chang ZQ, Serra CC (2014) A microfluidic-assisted fabrication of size-controlled porose CeO2 microspheres as an analog production of nuclear fuel beads. Adv Sci Technol 94:55–68
Hunt RD, Collins JL, Cowell BS (2017) Use of boiled hexamethylenetetramine and urea to increase the porosity of cerium dioxide microspheres formed in the internal gelation process. J Nucl Mater 492:1–5
Da Silva MFP, Matos JR, Isolani PC (2008) Synthesis, characterization and thermal analysis of 1:1 and 2:3 lanthanide(III) citrates. J Therm Anal Calorim 94:305–311
Da Silva MFP, De Souza Carvalho FM, Da Silva Tereza M, De Abreu Fantini MC, Isolani PC (2010) The role of citrate precursors on the morphology of lanthanide oxide obtained by thermal decomposition. J Therm Anal Calorim 99:385–390
Spaulding L, Brittain HG (1983) Intermolecular energy transfer between lanthanide complexes.8. Tb(III) donor and Eu(III)acceptor complexes of citric acid. J Lumin 28:385–394
Popa M, Kakihana M (2001) Praseodymium oxide formation by thermal decomposition of a praseodymium complex. Solid State Ionics 141–142:265–272
Collins et al. (2013) Formulation and method for preparing gels comprising hydrous cerium oxide. U.S. Patent No. 8436052B2
Wood S, Palmer D, Wesolowski D, Bénézeth P (2002) The aqueous geochemistry of the rare earth elements and yttrium. Part XI. The solubility of Nd (OH)3 and hydrolysis of Nd3+ from 30 to 290 °C at saturated water vapor pressure with in-situ pHm measurement. Geochem Soc Spec Publ 7:229–256
Acknowledgements
This work was financially supported by the Key Program for International S&T Cooperation Projects of China (No. 2016YFE0100700), National Natural Science Foundation of China(No. 51420105006), and “The Thirteenth Five-Year Plan” Discipline Construction Foundation of Tsinghua University (No. 2017HYYXKJS1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, X., Ma, J., Zhou, X. et al. Fabrication of CeO2–Nd2O3 microspheres by internal gelation process using M(OH)m and [MCit∙xH2O] (M=Ce3+, Ce4+, and Nd3+) as precursors. J Sol-Gel Sci Technol 92, 66–74 (2019). https://doi.org/10.1007/s10971-019-05058-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05058-4