Abstract
Сalcium aluminate Ca12Al14O33 and phosphors based on it were obtained using sol–gel technology. Complex studies were used to identify the dynamics of phase and structural transformations in the synthesis of Ca12Al14O33. The main stages of the calcium aluminate formation were determined using thermal analysis, IR spectroscopy and X-ray phase analysis. Solid-phase interaction in the formation of Ca12Al14O33 includes the steps of obtaining calcium carbonate and amorphous alumina (800 °C), aluminates of composition Ca3Al2O6 and CaAl2O4 (900–1000 °C), and Ca5Al6O14 and Ca4Al6O13 (1000–1100 °C). Ca12Al14O33 begins to form at 1100 °C. The graphic image of the structure was constructed according to the obtained atomic coordinates using the ReX Powder diffraction program. Luminescent properties of the phosphors of the composition Ca12−(0.05+x)Al14O33:NdxEu0.05 (x = 0.025–0.1) were investigated. It was determined that the highest intensity of luminescence is achieved at x = 0.05. With a further increase in the content of neodyum, there was a concentration quenching of the luminescence.

Highlights
-
Сalcium aluminate Ca12Al14O33 and phosphors based on it was obtained using sol–gel technology.
-
Based on thermal analysis, IR spectroscopy and X-ray phase analysis, scheme of intermediate products transformation during synthesis was proposed.
-
The highest intensity of luminescent was reached at a neodymium(III) content of 0.05 mol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Until now, white LEDs, which were obtained by combining a blue LED chip and a yellow phosphor based on an yttrium aluminum garnet, had been popular. In recent years, there has been a tendency to replace them with LEDs made on the basis of a combination of ultraviolet LED chips and trichromatic crystal phosphors. Red phosphor shows lower luminescence efficiency compared with blue and green phosphor among trichromatic phosphors. Therefore, at present, the development of red phosphors, which have good photoluminescent characteristics, high thermal and chemical stability, are efficiently excited by UV radiation is important today [1,2,3].
Alkaline earth metal aluminates have a diverse chemical composition and numerous crystalline structures [4,5,6,7,8]. They are used as matrices for obtaining effective crystal phosphors. Ions of trivalent europium are used as an activator ion. They emit an intense red light corresponding to the transition 5D0 → 7F2 when they are in a non-centrosymmetric environment [9]. The phase composition, crystal structure, and surface morphology of the luminescent matrix strongly depend on the calcination temperature and the ratio of Ca2+:Al3+ in the initial reaction mixture. This has a significant effect on the properties of the final product. It is known that phosphors based on alkaline earth metal aluminates, with the same M2+:Al3+ ratio in equivalent structures, have a radiation intensity that increases in the series Ca → Ba → Sr → Mg-containing phosphors [10, 11].
One of the most promising substances for obtaining effective red phosphors are cubic calcium aluminates of the composition Ca3Al2O6 and Ca12Al14O33, activated by europium (III) ions [12,13,14,15,16]. The crystal structure of Ca12Al14O33 consists of hollow formations with the inclusion of free oxygen ions. It is prompting suggestions that intensity of the emission of the europium ion will increase as a result of co-activation of neodymium (III) ions [17].
There are many ways to obtain aluminate systems, including the method of solid-phase synthesis, the method of chemical coprecipitation, the method of combustion, sol–gel method. At present sol–gel method is widely used. It is one of the most effective methods for obtaining materials with different properties [18,19,20,21]. Sol–gel method mainly differs from the others, it provides a homogeneous mixing of the starting components at the molecular level. It is achieved by dissolving of salts and oxides of the starting substances. This method allows us to achieve a reduction in energy costs. The product turns out high degree of purity at all stages of synthesis. Products are characterized by monophasic crystalline structure, strictly stoichiometric composition, absence of extraneous phases [22,23,24,25,26].
The purpose of the work was to study the phase formation and the formation of the structure of calcium aluminate of the composition Ca12Al14O33 and the preparation of phosphors based on it activated by europium (III) and neodymium (III) ions.
2 Experimental
2.1 Materials preparation
Сalcium nitrate tetrahydrate (Vekton, Russia), aluminum nitrate nonahydrate (LLC “NPF Nevsky chemist”, Russia) and citric acid monohydrate (LLC “CITROBEL”, Russia) was used as initial materials to synthesize Ca12Al14O33. A molar ratio of Ca:Al:H4Cit was 12:14:26. The luminescent materials Ca12−(0.05+x)Al14O33:NdxEu0.05 were obtained by introducing an ions of europium and neodymium. The molar ratio of the initial reagents is shown in Table 1. A certain amount of Eu2O3 and Nd2O3 was dissolved in the nitric acid (BIOMEDHIM, Russia). Salts were dissolved in the minimum amount of distilled water. The resulting clear solutions were mixed together and stirred for 1.5 h using a magnetic stirrer. Yellowish solution was subjected to heat treatment at 130 °С in the drying oven (SNOL 58/350, Lithuania) for 5 h. The resulting xerogel was ground and calcined in the muffle furnace (SNOL 6/1300, Lithuania) at different temperature (from 200 to 1200 °C) for 3 h. Scheme of the synthesis is shown in Fig. 1.
2.2 Materials characterization
For researching of processes which proceed at calcium aluminate formation we used a complex of physical and chemical research techniques:
-
thermal analysis was done using a NETZSCH STA 449C thermoanalyser (“NETZSCH” Germany) at a rate of 5°/min in air, the temperature range was from 25 to 1000 °C;
-
XRD was done using Rigaku MiniFlex 600 (Japan) (Cu Kα radiation λ = 1.5406 Å) at room temperature with the range of angles 2θ from 3 to 80°) at the scanning speed of 2°/min. The phase identification was carried out using PDF-2 database. The structure refinement was made by the Rietveld method using the ReX Powder diffraction program [27,28,29];
-
the crystal structure model is constructed using the VESTA program [30];
-
IR spectroscopy was done by IR spectrometer Agilent Technologies Cary 600 Series FTIR Spectrometer (made in USA). Decoding of IR spectra was carried out according to the literature [8, 12, 31, 32];
-
the luminescent properties were investigated using a spectrofluorometer Solar CM 2203 (Belarus).
3 Result and discussion
We defined the main stages of the synthesis process, the structure of prepared powders and the nature of the bonding using Thermal analysis, XRD and IR spectroscopy.
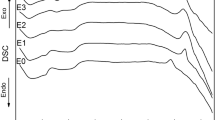
Analysis of TG and DSC curves allowed to see the main stages of formation of calcium aluminate in the temperature range from 25 to 1000 °C (Fig. 2).
The thermogram shows that some processes overlap each other. At the first stage there is a solvent remove from the surface of the sample, it is accompanied by a small endothermic effect and a small value of activation energy. The activation energy of this process is 58 kJ/mol. It indicates the course of the physical process. Activation energy calculated by Metzger–Horowitz method [25].
At the temperature ranges from 205 to 450 °C there is a sharp decrease of mass of the sample. It is accompanied by a large exothermic effect. The activation energy is 195 kJ/mol. It indicates the course of chemical processes, such as the decomposition of the organic framework. At further temperature increase there is a decomposition of a polymeric complex and oxidation of organic matters. It is accompanied by some exothermic effects.
At temperatures above 700, aluminates are formed. These processes are accompanied by high values of activation energy. The activation energy is about 700 kJ/mol.
We received samples at a temperature of 130, 300, 450, 600, 800, 900, 1000, 1100, and 1200 °C in order to describe in detail the processes that occur at each stage and investigated the received samples using IR spectrometric and X-ray phase analyzes.
Figure 3 shows the IR spectra of Ca12Al14O33 precursors. According to the data of IR spectroscopy, we can say that all the M–O bonds are formed during the maturation process of the solution. In the future, we observe only the growth of the polymer.
At low temperatures from 130 to 300 °C in the regions from 1410 to 1340 cm−1 and from 860 to 800 cm−1, there are characteristic bands belonging to the nitrate ion. In samples obtained up to 500 °C, there are bands in the range from 1610 to 1550 cm−1. They are associated with stretching vibrations of the carboxyl group in citrate complexes. With increasing the temperature, the vibrations of the carboxyl group and the vibrations of the C–O bond in the carboxyl group disappear. It indicates the decomposition of citrate complexes.
When the initial solution passes into the gel, there is characteristic fluctuation of the O–C–O in 1074 cm−1. It is due to the polycondensation process. At temperatures above 900, we can see bands in the low-frequency region from 900 to 500 cm−1 related to vibrations of the Ca–O–Al bond. This bond forms the basis of the calcium aluminate crystal lattice.
In the samples obtained during annealing in the temperature range from 600 to 800 °С there are bands in the region of 1450–1410 cm−1, corresponding to the carbonate ion. X-ray phase data confirm the presence of CaCO3 in the samples.
The strong broad band absorptions at around 800 cm−1 are attributed to [AlO4] tetrahedra stretching vibration, which are composed by two absorption peaks at 850 cm−1 for “condensed” AlO4 and at 773 cm−1 for “isolated” AlO4 tetrahedra [33]. Those results demonstrate there are two [AlO4] tetrahedral structures in the lattice, which is accordance with the obtained structure in the Ca12Al14O33 unit cell [34]. The peaks located 617 cm−1 and 574 cm−1 should derive from characteristic vibration absorption of Al–O bonds.
X-ray phase analysis data of the Ca12Al14O33 precursors calcined at 800–1200 °С are presented in Table 2 and in Figs. 4 and 5.
Until the temperature of 700 °C, the samples are X-ray amorphous. Heating the precursors at 800 °C for 3 h produces a mixture of crystalline calcium carbonate and amorphous alumina. Noncrystalline alumina is undetect by diffraction methods, therefore evidence of its existence is indirect. Heating at 900 °C gives a mixture of Ca3Al2O6 and CaAl2O4. Mass ratio of aluminates is practically equal. Increasing calcination temperature to 1000 °C, the content of the phases Ca3Al2O6 and CaAl2O4 significantly decreases. New phases Ca5Al6O14 and Ca4Al6O13 appear. The peaks of these aluminates are clearly visible on X-ray diffraction patterns. Ca12Al14O33 begins to form at 1100 °C, but the main phase is Ca5Al6O14. The content of Ca4Al6O13 and Ca3Al2O6 at this temperature significantly decreases. At 1200 °C, Ca12Al14O33 becomes the main phase. An insignificant content of Ca5Al6O14 and Ca4Al6O13 is also observed. These intermediates of the synthesis also have been discovered by other researchers [25]. Based on the experiment, the following transformation scheme was proposed:
(1) The precursors decompose to CaCO3 and Al2O3.
(2) Calcium carbonate is decomposed to CaO and CO2. It is confirmed by the results of thermal analysis (we can see endothermic effect on the DSC curve and mass reduction on the TG curve):
(3) At 900 °C, monoaluminate and tricalcium aluminate are formed:
(4) At 1000 °C, monoaluminate and tricalcium aluminate are converted to Ca5Al6O14 and Ca4Al6O13. The following transformations are possible:
The formation of these aluminates is most likely due to the similarity of the structures Ca3Al2O6 and Ca4Al6O13, and CaAl2O4 and Ca5Al6O14. Although it is possible that other aluminates are formed:
(5) At 1100 °C, Ca5Al6O14 becomes the main phase:
It is possibly:
The main product begins to form:
It is possibly:
(6) At 1200 °C, cubic Ca12Al14O33 is formed.
With increasing synthesis temperature, the following scheme is implemented:
The structure refinement was made by the Rietveld method using the ReX Powder diffraction program. The calculation results showed that the space group I4\(\bar 3\)d, a = 12.043 Å, Rp = 0.168136, Rexp = 0.168202, and Rwp = 0.223990. The graphic image of the structure was constructed according to the obtained atomic coordinates.
Ca12Al14O33 has a complex structure composed of hollow spheres (Fig. 6). It possesses a framework composed of aluminum-oxygen tetrahedra [AlO4]. Tetrahedra are interconnected through oxygen atoms and partially through bridging calcium cations, forming eight-membered rings. The structure includes one type of calcium atoms (Ca1), two types of aluminum atoms (Al1 and Al2) in a tetrahedral environment of oxygen and three types of oxygen (O1, O2, and O3). They are distinguished by their surroundings. O1 are coordinated by the aluminum atom Al2; O1 and O2 are coordinated by the aluminum atom Al1. They form aluminum-oxygen tetrahedra, herewith tetrahedron containing O2 is distorted. O3 form anions of unbound oxygen. Such anions O22− are located inside hollow spheres.
Phosphors based on Ca12Al14O33, activated by europium (III) and neodymium (III) ions were obtained at a temperature of 1200 °С.
We obtained excitation (Fig. 7) and photoluminescence (Fig. 8) spectra for samples with different content of neodymium in Ca12Al14O33 (n(Nd3+): 1—0; 2—0.025; 3—0.05; 4—0.075; 5—0.1 mol).
An intense band with λmax = 250–260 nm is observed in the short-wave region of the excitation spectrum. It indicates the transfer of excitation energy from the O2– ion to the Eu3+ ion. Therefore, we used an excitation wavelength of 254 nm in obtaining luminescence spectra. In the luminescence spectra of phosphors containing europium(III) and neodymium(III) ions, several narrow bands with a wavelength of 618 nm are observed. It corresponds to the red region of the spectrum and indicates the occurrence of europium(III) ion in the structure of calcium aluminate in the trivalent state. The luminescence spectrum consists of characteristic narrow emission lines of the Eu3+ ion, corresponding to the transitions of electrons inside the 4f shell. The most intense is the induced electric dipole transition 5D0–7F2 with a maximum at a wavelength of 618 nm, then 5D0–7F4 with a wavelength of 704 nm. The magnetic dipole transition 5D0–7F1 has the lowest intensity (λmax = 595 nm) [17].
The presence of neodymium(III) ions in the structure of calcium aluminate affects the radiation intensity of luminescence centers, which include europium(III). The highest intensity is reached at a neodymium content of 0.05 mol. With increasing concentration of the Nd3+ ion, concentration quenching occurs and the radiation intensity decreases.
4 Conclusion
Сalcium aluminate with the composition Ca12Al14O33 and phosphors based on it, containing europium(III) (0.05 mol) and neodymium(III) (from 0.025 to 0.1 mol) were obtained using sol–gel technology.
The main stages of the formation of calcium aluminate were determined using thermal analysis, IR spectroscopy and X-ray phase analysis. Solid-phase interaction in the formation of Ca12Al14O33 includes the steps of obtaining calcium carbonate and amorphous alumina (800 °C), aluminates of composition Ca3Al2O6 and CaAl2O4 (900–1000 °C), and Ca5Al6O14 and Ca4Al6O13 (1000–1100 °C). Ca12Al14O33 begins to form at 1100 °C.
The graphic image of the structure was constructed according to the obtained atomic coordinates using the ReX Powder diffraction program.
Luminescent properties of the phosphors of the composition Ca12−(0.05+x)Al14O33:NdxEu0.05 (x = 0.025–0.1) were investigated. It was determined that the highest intensity of luminescence is achieved at x = 0.05. With a further increase in the content of neodyum there is a concentration quenching of the luminescence.
References
Li H, Noh HM, Moon BK, Choi BC, Jeong JH, Jang K, Lee HS, Yi SS (2013) Wide-band excited Y6(W, Mo)0.5O12:Eu red phosphor for white light emitting diode: structure evolution, photoluminescence properties, and energy transfer mechanisms involved. Inorg Chem 52(19):11210–11217
Li YT, Liu XH (2014) Structure and luminescence properties of Ba3WO6:Eu3+ nanowire phosphors obtained by conventional solid-state reaction method. Opt Mater 38:211–216
Jin C, Ma HX, Liu QB, Li X, Liu PF (2014) Photoluminescence properties of the high-brightness Eu3+-doped KNaCa2(PO4)2 phosphors. Spectrochim Acta A 122:767–771
Singh V, Borkotoky S, Murali A, Rao JL, Gundu Rao TK, Dhoble SJ (2015) Electron paramagnetic resonance and photoluminescence investigation on ultraviolet-emitting gadolinium-ion-doped CaAl12O19 phosphors. Spectrochim Acta A 139:1–6
Puchalska M, Sobczyk M, Targowska J, Watras A, Zych E (2016) Infrared and cooperative luminescence in Yb3+ doped calcium aluminate CaAl4O7. J Lumin 143:503–509
Pakhnutova EA, Mishenina LN, Selyunina LA, Belyaninova TV, Slizhov YG (2018) Nitrate-citrate sol-gel synthesis of hydrated calcium aluminate and sorption materials on its basis. Russ J Appl Chem 91(6):899–907
Genga G, Myersa RJ, Yud YS, Shapirod DA, Winarskie R, Levitzf PE, Kilcoyned DAL, Paulo JM (2018) Synchrotron X-ray nanotomographic and spectromicroscopic study of thericalcium aluminate hydration in the presence of gypsum. Cement and Concrete Research 111:130–137
Pan X, Zhang D, Wu Y, Yu H (2018) Synthesis and characterization of calcium aluminate compounds from gehlenite by high-temperature solid-state reaction. Ceram Int 44:13544–13550
Hussain A, Mehmood S, Rasool MN, Aryal, Rulis P, Ching WY (2016) Electronic structure, mechanical, and optical properties of CaO∙Al2O3 system: a first principles approach. Indian J Phys 8:917–929
Park K, Pi JW, Hakeem DA (2016) Effect of alkaline metal ions on the photoluminescence properties of Eu3+-doped Ca3Al2O6 phosphors. J Rare Earths 34(12):1193–1198
Liang CJ, Siao HY (2016) Luminescence behaviors of Eu- and Dy-codoped alkaline earth metal aluminate phosphors through potassium carbonate coprecipitation. Mater Chem Phys 177:429–436
Liang CJ, Siao HY (2017) Effects of calcining temperatures of Eu2+ and Dy3+ ion-codoped calcia-alumina binary compounds on their phase transition and luminescence properties. Mater Chem Phys 193:117–128
Yadav RV, Yadav RS, Bahadur A, Rai SB (2016) Down shifting and quantum cutting from Eu3+, Yb3+ co-doped Ca12Al14O33 phosphor: a dual mode emitting material. RSC Adv 6:9049–9056
Rashad MM, Mostafa AG, Rayan DA (2016) Structural and optical properties of nanocrystalline mayenite Ca12Al14O33 powders synthesized using a novel route. J Mater Sci: Mater Electron 27:2614–2623
Gao X, Lei L, Lv C, Sun Y, Zheng H, Cui Y (2008) Preparation and photoluminescence property of a loose powder Ca3Al2O6:Eu3+ by calcination of a layered double hydroxide precursor. J Solid State Chem 181:1776–1781
Barros BS, de Oliveira RS, Kulesza J, Melo VRM, Melo DMA, Alves JrS (2015) Reddish-orange Ca3-xAl2O6:xEu3+ nanophosphors: Fast synthesis and photophysical properties. J Phys Chem Solids 78:90–94
Zhang J, Zhangb Z, Wanga T, Hao W (2003) Preparation and characterization of a new long afterglow indigo phosphor Ca12Al14O33:Nd,Eu. Mater Lett 57:4315–4318
Bakhmetyev VV, Minakova TS, Mjakin SV, Sychov MM, Ringuede A (2016) Synthesis and surface characterization of nanosized Y2O3:Eu and YAG:Eu luminescent phosphors which are useful in photodynamic therapy of cancer. Eur J Nanomed 8(4):173–184
Mal’chik AG, Kuznetsova SA, Kryuchkova SO, Kozik VV (2017) Sol–gel synthesis of Ta2O5–SiO2 composites from tantalum(V) chloride and tetraethyl orthosilicate in ethanol. Inorg Mater 53(9):994–1003
Agliullin MR, Grigor’eva NG, Danilova IG, Magaev OV, Vodyankina OV (2015) Template-free sol-gel synthesis of catalytically active mesoporous aluminosilicates. Kinet Catal 56(4):501–508
Mishenina LN, Selyunina LA, Botvina TM (2015) Synthesis and characteristics of CaAl2O4:Eu3+ phosphor prepared by zol-gel method. Key Eng Mater 670:95–100
Aitasalo T, Hölsä J, Jungner H et al. (2002) Comparison of sol-gel and solid-state prepared Eu2+ doped calcium aluminates. J Mater Sci 20(1):15–20
Mandić V, Kurajica S (2015) The influence of solvents on sol–gel derived calcium aluminate. Mater Sci Semicond Process https://doi.org/10.1016/j.mssp.2015.01.004
Choi SW, Hong H (2010) Size and morphology control by planetary ball milling in CaAl2O4:Eu2+ phosphors prepared by Pechini method and their luminescence properties. J Mater Sci Eng B 171:69–72
Yuan X, Xu YB, He Y (2007) Synthesis of Ca3Al2O6 via citric acid precursor. J Mater Sci Eng, A 447:142–145
Botvina TM, Botvin VV, Mishenina LN, Selyunina LA (2018) Synthesis of Calcium Aluminate-Based Luminophores by the Citrate Nitrate Sol–Gel Process. Russ J Inorg Chem 63(10):1262–1267
Bortolotti M, Lonardelli I (2013) ReX.Cell: a user-friendly program for powder diffraction indexing. J Appl Cryst 46:259–261
Boultif A, Louër D (2004) Powder pattern indexing with the dichotomy method. J Appl Cryst 37:724–731
Visser JW (1969) A fully automatic program for finding the unit cell from powder data. J Appl Cryst 2:89–95
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44:1272–1276
Wang R, Yang H, Lu Y, Kanamori K, Nakanishi K, Guo X (2017) Synthesis, reduction, and electrical properties of macroporous monolithic mayenite electrides with high porosity. ACS Omega 2:8148–8155
Liu1 R, Yan Y, Ma Ch (2018) Self-propagating combustion synthesis, luminescent properties and photocatalytic activities of pure Ca12Al14O33:Tb3+(Sm3+). https://doi.org/10.3389/fchem.2018.00069
Tolkacheva AS, Shkerin SN, Plaksin SV, Vovkotrub EG, Bulanin KM, Kochedykov VA, Ordinartsev DP, Gyrdasova OI, Molchanova NG (2011) Synthesis of dense ceramics of single-phase mayenite (Ca12Al14O32)O. Russ J Appl Chem 84(6):907–911
Boysen H, Lerch M, Stysb A, Senyshyn A (2007) Structure and oxygen mobility in mayenite (Ca12Al14O33): a high-temperature neutron powder diffraction study. Acta Cryst B63:675–682
Acknowledgements
The work was performed as part of the state assignment of the Ministry of Education and Science of the Russian Federation for project No. 4.9607.2017/8.9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mishenina, L., Selyunina, L., Broslavskaya, T. et al. Phase formation and luminescent properties of Сa12Al14O33:Eu, Nd prepared by sol–gel method. J Sol-Gel Sci Technol 92, 515–522 (2019). https://doi.org/10.1007/s10971-019-04992-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-04992-7