Abstract
In this study, stearic acid/silica phase change composites were prepared by the sol-gel method using stearic acid as phase change materials (PCMs). The effects of mass fraction of stearic acid were comprehensively investigated. The structures and thermal properties of the obtained composites were characterized by various methods, including scanning electron microscopy (SEM), differential scanning calorimetry (DSC), leakage tests, and thermogravimetry analysis (TG). The results indicated that composite containing 76% stearic acid had the best thermal properties and low mass leakage, making 76% stearic acid as the maximum content that silica matrix could protect in the composites. The latter was further confirmed by morphological analyses of the silica matrix. Silica matrix exhibited spherical particle clusters, following big–small–big–small size pattern as stearic acid rose. The composite with 76% stearic acid was at the key point of change in particle size. These findings look promising for future to prepare silica-based phase change composites with good thermal properties easily.

Highlights
-
The composite containing 76% stearic acid showed good thermal properties.
-
Silica particle size changed with stearic acid content in composites.
-
The composite with 76% stearic acid was at the key point of change in particle size.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phase change materials (PCMs) can absorb or release large amounts of latent heat. Hence, they are often used as heat storage materials for different purposes such as seasonal energy storage, thermo-regulated textile, and energy-saving building materials [1,2,3,4]. PCMs are usually divided into two kinds from chemical composition: organic and inorganic PCMs [1]. Organic PCMs, such as paraffin, polyethylene glycol (PEG) and fatty acids are the most commonly used materials due to their suitable melting temperatures, high energy storage abilities, and chemical inertness [5, 6]. Among investigated organic PCMs, paraffin and fatty acids have attracted increasing interest due to their low cost. However, leakage during thermal cycling limits their usage since this decreases the effective enthalpy and shortens lifetime of the material [7]. Therefore, numerous form-stable PCM composites and encapsulated PCMs have been investigated to overcome the leakage issue [7,8,9,10].
Silica [11, 12], expanded graphite [13, 14], Kaolin [15], diatomite [16], and gypum [3] are used as support materials in form-stable PCM composites for their porous structure. PCMs are embedded in the support materials by different means, including sol-gel [17,18,19,20,21,22], vacuum adsorption [14, 23], and polymerization [24, 25]. By vacuum adsorption method, the mass of PCMs adsorbed into support materials relies on the support materials pores volume. It is hard to increase the mass of adsorbed PCMs under this limitation. But there are more possibilities to change the pore structure in composites by using sol-gel and polymerization methods. Therefore, silica-based phase change composites are synthesized by mature sol-gel technology [17,18,19,20,21,22]. On the other hand, in this process, silica sol is first mixed with different PCMs to embed them in the silica framework after gelation, which is simple and easy to do [26]. Silica is one of the components of building materials, and silica-based PCMs composites materials could be given priority in building heat-regulating materials [27].
However, the properties of form-stable PCM composites and the appropriate mass content of PCMs in composites are different with the same sol-gel method. Ma et al. [21] synthesized lauric acid/silica composites using maximum mass of lauric acid of 69.1%. And lauric acid was uniformly imbedded into silicon dioxide without melted leakage from the composites. Li et al. [19] prepared paraffin/SiO2 composites by the sol-gel method using maximum mass content of paraffin of 53.5%, with latent heat 112.8 kJ/kg. Silicone gel was formed and paraffin was dispersed in the porous network. Fang et al. [20] prepared palmitic acid/SiO2 composite with latent heat 85.11 kJ/kg. The maximum mass percentage of palmitic acid in the composites was estimated to 41.1%, and the leakage of composites may occur with the increasing mass of palmitic acid. Li et al. [22] prepared the shape-stabilized paraffin/silicon dioxide composite by using sol-gel method. The maximum mass percentage of paraffin dispersed into the composites was up to 92.1%, and there was no leakage of the paraffin from the composites.
In this view, several studies reported silica-based PCMs composites with relevant properties and the maximum PCMs content, the causes behind the results were rarely discussed. So it is especially important to discover the rule of formation process in preparing silica-based phase change composites. In our previous study, silica-based phase change composites containing organic PCMs of stearic acid, paraffin and polyethylene glycol were prepared by the sol-gel process [8]. During gelation, the three PCMs made the pore size of silica framework different. And this led to different thermal properties of the composites. Among them, the prepared stearic acid/silica composites showed good thermal properties. Therefore, a further and systematical research about stearic acid/silica composites should be proceeded to discover the rule of form-stable process.
In this study, several stearic acid/silica phase change composites were prepared using the sol-gel method. The maximum mass content of stearic acid present in PCM composites, leakage tests, and silica structure were examined. The relationship between thermal properties of the composites and silica structure, and the formation of silica structure in composites were explained.
2 Experimental
2.1 Materials
Tetraethyl silicate (TEOS, Analytical Reagent) was purchased from Tianjin Kemiou Chemical Reagent, China. Stearic acid (CH3(CH2)16COOH, Analytical Reagent) was obtained from Guangzhou Jinhua Chemical Reagent, China. Hydrochloric acid (HCl, Analytical Reagent, 36~38wt.%), ammonium hydroxide (NH3·H2O, Analytical Reagent, 28 wt.%) and ethanol (CH3CH2OH, Analytical Reagent, Chengdu Institute of Chemical Reagents, China) were used as received without further purification. Deionized water was used for preparation of all aqueous phases.
2.2 Composite preparation
First, silica sol was prepared by the following procedure: TEOS (60 mL), ethanol (120 mL) and distilled water (0.5 mL) were mixed in a 500 mL flask under magnetic stirring. Next, hydrochloric acid was added as catalyst and pH of the mixture was adjusted to 1–2. The mixture was then kept at 60 °C for 6 h to obtain the silica sol.
Second, phase change composites were prepared by the following route: silica sol (4.5 g) and ethanol (4 g) were mixed with stearic acid (1, 2, 2.5, 3.5, 5, 6, 7, and 9 g) in respective beakers. Ammonium hydroxide was then added to the mixture. After stirring at 800 rpm in water bath for 0.5 h at 60 °C, gels were obtained. Next, the composite gels were dried at 80 °C for 24 h to yield stearic acid/silica composites. The composites were ground in an agate mortar and marked as SAS1, SAS2, SAS3, SAS4, SAS5, SAS6, SAS7 and SAS8, corresponding to stearic acid contents of 38, 56, 61, 69, 76, 79, 81 and 85%, respectively. And the content of stearic acid and silica in SAS composites were showed in Table 1.
2.3 Characterization
The phase change properties in terms of melting/freezing points and latent heats were obtained by differential scanning calorimetry (DSC, TA, Q-100) from 25 to 70 °C at heating/cooling rate of 5 °C min−1 under argon atmosphere. The chemical compositions of SAS composites were tested by Fourier transform Infrared (FTIR, Nicolet iS5) spectroscopy. The morphologies were characterized by scanning electron microscopy (SEM, Hitachi TM-3000). The thermal stabilities of SAS composites were obtained by a thermogravimetry analyzer (TG, TA Q-500) at the scanning rate of 10 °C min-1, temperature range of 50-600 °C, and under argon atmosphere.
The leakage tests could provide information on changes in composite thermal properties. The composites were first weighed then placed on filter paper. After heating at 80 °C for 20 min followed by cooling to room temperature, the composites were weighed again and mass loss was calculated. Next, the composites were placed on other filter papers to run other thermal cycles. The above procedure was performed consecutively until mass loss was close to zero.
3 Results and discussion
3.1 Chemical characterization of composites
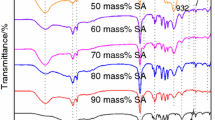
Figure 1 shows the FTIR spectra of stearic acid, SiO2, and prepared SAS composites with various mass fractions of stearic acid. The spectrum of pure SiO2 revealed extra peaks at 1079 cm-1 and 795 cm-1, assigned to asymmetric and symmetric stretching vibration of Si–O–Si group, respectively. The broad peak at 3436 cm-1 was attributed to bending vibration of Si–OH. The peaks at 2917 cm-1 and 2849 cm-1 in the spectrum of stearic acid originated from C–H stretching vibrations of methyl and methylene groups, respectively. The peaks at 1472 cm-1 and 1463 cm-1 were attributed to bending vibrations of methylene group, and those at 1704 cm-1 and 1303 cm-1 corresponded to stretching vibrations and in-plane bending vibrations of C=O group and –OH, respectively. Overall, all the characteristic peaks of stearic acid and SiO2 were present in spectra of the composites. Also, no new peaks appeared in spectra of the composites, indicating the physical mixture of stearic acid and SiO2 during gelation of silica sol.
3.2 Thermal properties of composites
3.2.1 DSC analysis
Figure 2 illustrates the DSC curves of stearic acid and SAS composites. The heat absorption and release properties of the composites and stearic acid are listed in Table 2. The latent heat values of the composites were compared to those of stearic acid, and the effective PCM mass percentage in the composites was calculated by Eq. (1),
where ΔHcomposites and ΔHstearic acid (170.3 J g-1) are the melting enthalpies of the composites and pure stearic acid measured by DSC, respectively.
As can be seen in Fig. 2, all composites exhibited the endothermic and exothermic peaks of stearic acid. As mass fraction of stearic acid rose in the composites, the endothermic and exothermic peak intensities strengthened. Figure 2a estimated the melting temperature of SAS composites between 53.30 °C and 55.30 °C. A reduction in melting temperature of SAS composites ranging from 1.3 to 3.3 °C was noticed when compared to pure stearic acid. The most of effective stearic acid content calculated by Eq. (1) was below theoretical stearic acid content, except for SAS3, SAS4, and SAS8 in Table 2. The reduction in the effective PCM mass percentage was caused by confinement effect of silica mesopores [8, 21, 28]. And Schmidt et al. [29] offered the existence of “a nonfreezing layer”, which acted physically as part of the pore wall, to decrease the latent heat [30]. However, increment in effective PCM mass percentage would be an interesting phenomenon to study. These findings may indicate that thermal enthalpy of stearic acid in the composites was affected by silica structure.
3.2.2 TG testing
The thermal stabilities of stearic acid and SAS composites were evaluated by TG analysis and the results are gathered in Fig. 3. A one-step mass loss of pure stearic acid was observed, with onset weight loss temperature of 180 °C. By comparison, two-step mass losses were recorded for SAS composites. From 200 °C to 300 °C, the mass loss in SAS composites was caused by vaporization of stearic acid. From 300 °C to 500 °C, the mass loss was linked to water loss from further gelatinization. The inset revealed that onset weight loss of SAS composites shifted to high temperatures, especially for SAS1, SAS2, and SAS5. Obviously, the thermal stability of SAS1, SAS2 and SAS5 composites enhanced more than the other SAS composites. Under the action of surface tension and capillary force, the vaporization temperature of stearic acid was retarded. When stearic acid content was below 76%, the effect of capillary force was more significant.
3.2.3 Leakage testing
Leakage testing of the composites would provide information related to effect of composite content on thermal stability. Figure 4 shows photos of the composites after the first thermal treatment. Leakage of stearic acid can clearly be seen from the filter paper but leakage mass among samples differed. And Fig. 5 depicts the mass loss rate of SAS composites after each thermal cycle. SAS1, SAS2, SAS3 and SAS5 composites showed gentle variation trends, with total mass losses of less than 10%. However, SAS4, SAS6, SAS7 and SAS8 illustrated distinct changes, with total mass losses exceeding 40%. As stearic acid content rose in the composites, the mass loss increased quickly after SAS5 (76% stearic acid). That was to say stearic acid content was near 76%, which was the maximum content that silica matrix could protect in the composites.
3.3 Morphology of silica in composites
The stearic acid present in the composites was extracted by ethanol for more than three times, until stearic acid could no longer be detected in silica matrix by FTIR spectroscopy. The remaining structure of silica was analyzed by SEM and the results are depicted in Fig. 6. The silica matrix exhibited spherical particle clusters with different sizes. The spherical particle sizes followed the pattern big–small–big–small as stearic acid content rose. On the other hand, as stearic acid increased, the spherical particle size of silica for both SAS1 to SAS5 and SAS6 to SAS8 reduced but the decreasing trends were not continuous. SAS5 was shown at the inflection point of change in particle size. Obviously, the gelation morphology of silica was influenced by stearic acid content in the mixed solution, and 76% stearic acid content was at the critical point of change in particle size.
Based on the above results, the change in silica morphology is shown in Fig. 7. At low contents of stearic acid solutions, stearic acid acted as dispersed phase and silica sol as continuous phase in the mixed solution. The formation of mixed solution was broken at stearic acid contents near 76% (SAS5). In other words, phase inversion occurred for stearic acid contents up to 76%, and stearic acid changed from dispersion phase to continuous phase. Therefore, the spherical particle size of silica decreased as stearic acid rose at contents below 76%. After phase inversion, the spherical particle size of silica changed to form new point. The change in morphology of continuous or dispersion phases as a function of content were widely investigated in emulsion [31, 32]. With the increment of dispersion phase, the particle size of dispersion phase became large and incomplete phase inversion was achieved through the coalescence [32]. And their morphology change trends were similar to the change of silica morphology. But near the phase inversion, the silica morphology became complex, which led to anomalous properties of SAS4 composite such as the increment in effective PCM mass percentage and mass lose exceeding 40% of SAS4. And Fig.7 showed one of the most common processes of phase inversion.
Compared to TG data and leakage tests, SAS5 showed good thermal properties and low mass leakage. This indicated that stearic acid acted as dispersion phase, which would benefit the thermal properties of the composites in mixed solution. Near the point of phase inversion, the maximum content of stearic acid in composites was reached. This represented the maximum content of stearic acid, which silica matrix could embed and protect.
4 Conclusions
Stearic acid was used as PCM for thermal energy storage. Stearic acid/silica composites were prepared by the sol-gel method. The chemical properties, thermal characteristics and morphology of silica in the composites were studied. The FTIR spectral confirmed the chemical composition of SAS composites. The DSC results showed that thermal enthalpy of stearic acid in the composites was affected by silica content, and the most of effective stearic acid content was below theoretical stearic acid content. Leakage tests revealed the composites with low leakage at stearic acid mass contents below 76%, except for SAS4 composite. After extraction of stearic acid from the composites, silica matrix exhibited spherical particle clusters, following big–small–big–small size pattern as stearic acid rose. From structure viewpoint, a point of phase inversion of mixed solution probably occurred as stearic acid content rose, and near phase inversion stearic acid in composites reached maximum content at 76%, maximum content that the silica matrix could protect. The structure of SAS composites was different before and after phase inversion, which would be a key factor to affect the composites thermal properties.
References
Zalba B, Marın JM, Cabeza LF, Mehling H (2003) Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Appl Therm Eng 23:251–283
He F, Wang X, Wu D (2015) Phase-change characteristics and thermal performance of form-stable n-alkanes/silica composite phase change materials fabricated by sodium silicate precursor. Renew Energy 74:689–698
Karaipekli A, Sar A, Biçer A (2016) Thermal regulating performance of gypsum/(C18-C24) composite phase change material (CPCM) for building energy storage applications. Appl Therm Eng 107:55–62
Su W, Darkwa J, Kokogiannakis G (2017) Development of microencapsulated phase change material for solar thermal energy storage. Appl Therm Eng 112:1205–1212
Sánchez P, Sánchez-Fernandez MV, Romero A, Rodríguez JF, Sánchez-Silva L (2010) Development of thermo-regulating textiles using paraffin wax microcapsules. Thermochim Acta 498:16–21
Jin Z, Wang Y, Liu J, Yang Z (2008) Synthesis and properties of paraffin capsules as phase change materials. Polym (Guildf) 49:2903–2910
Yang D, Shi S, Xiong L et al. (2016) Paraffin/Palygorskite composite phase change materials for thermal energy storage. Sol Energy Mat. Sol Cells 144:228–234
Fu Z, Su L, Liu M, Li J, Li J, Zhang Z, Li B (2016) Confinement effect of silica mesopores on thermal behavior of phase change composites. J Sol-Gel Sci Technol 80:180–188
Zhang H, Wang X, Wu D (2010) Silica encapsulation of n-octadecane via sol-gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J Colloid Interface Sci 343:246–255
Cao L, Tang Y, Fang G (2015) Preparation and properties of shape-stabilized phase change materials based on fatty acid eutectics and cellulose composites for thermal energy storage. Energy 80:98–103
Luo R, Wang S, Wang T, Zhu C, Nomura T, Akiyama T (2015) Fabrication of paraffin@ SiO2 shape-stabilized composite phase change material via chemical precipitation method for building energy conservation. Energy Build 108:373–380
He L, Li J, Zhou C, Zhu H, Cao X, Tang B (2014) Phase change characteristics of shape-stabilized PEG/SiO2 composites using calcium chloride-assisted and temperature-assisted sol gel method. Sol Energy 103:448–455
Zhang Q, Wang H, Ling Z, Fang X, Zhang Z (2015) RT100/expand graphite composite phase change material with excellent structure stability, photo-thermal performance and good thermal reliability. Sol Energy Mat. Sol Cells 140:158–166
Zhang Z, Shi G, Wang S, Fang X, Liu X (2013) Thermal energy storage cement mortar containing n-octadecane/expanded graphite composite phase change material. Renew Energy 50:670–675
Memon SA, Lo TY, Shi X, Barbhuiya S, Cui H (2013) Preparation, characterization and thermal properties of Lauryl alcohol/Kaolin as novel form-stable composite phase change material for thermal energy storage in buildings. Appl Therm Eng 59:336–347
Kenisarin MM, Kenisarina KM (2012) Form-stable phase change materials for thermal energy storage. Renew Sust Energy Rev 16:1999–2040
Ma F, Zong X, Chen M, Li Y (2010) Preparation and properties of lauric acid/SiO2 phase change energy storage composite materials. J Mat Eng 4:15–17
Qian T, Li J, Ma H, Yang J (2015) The preparation of a green shape-stabilized composite phase change material of polyethylene glycol/SiO2 with enhanced thermal performance based on oil shale ash via temperature-assisted sol–gel method. Sol Energy Mat. Sol Cells 132:29–39
Li M, Wu Z, Tan J (2012) Properties of form-stable paraffin/silicon dioxide/expanded graphite phase change composites prepared by sol-gel method. Appl Energy 92:456–461
Fang G, Li H, Chen Z, Liu X (2011) Preparation and properties of palmitic acid/SiO2 composites with flame retardant as thermal energy storage materials. Sol Energy Mat. Sol Cells 95:1875–1881
Yang H, Feng L, Wang C, Zhao W, Li X (2012) Confinement effect of SiO2 framework on phase change of PEG in shape-stabilized PEG/SiO2 composites. Eur Polym J 48:803–810
Li H, Fang G, Liu X (2010) Synthesis of shape-stabilized paraffin/silicon dioxide composites as phase change material for thermal energy storage. J Mater Sci 45:1672–1676
Zong X, Cai Y, Sun G, Zhao Y, Huang F, Song L, Hu Y, Fong H, Wei Q (2015) Fabrication and characterization of electrospun SiO2 nanofibers absorbed with fatty acid eutectics for thermal energy storage/retrieval. Sol Energy Mater Sol Cells 132:183–190
Zhang Y, Wang L, Tang B, Lu R, Zhang S (2016) Form-stable phase change materials with high phase change enthalpy from the composite of paraffin and cross-linking phase change structure. Appl Energy 184:241–246
Sari A (2004) Form-stable paraffin/high density polyethylene composites as solid-liquid phase change material for thermal energy storage: preparation and thermal properties. Energy Convers Manag 45:2033–2042
Guo X, Zhang Q, Ding X, Shen Q, Wu C, Zhang L, Yang H (2016) Synthesis and application of several sol–gel-derived materials via sol–gel process combining with other technologies: a review. J Sol-Gel Sci Technol 79:328–358
Jeong SG, Jeon J, Cha J, Kim J, Kim S (2013) Preparation and evaluation of thermal enhanced silica fume by incorporating organic PCM, for application to concrete. Energy Build 62:190–195
Jackson CL, McKenna GB (1990) The melting behavior of organic materials confined in porous solids. J Chem Phys 93:9002
Schmidt R, Hansen EW, Stoecker M, Akporiaye D, Ellestad OH (1995) Pore size determination of MCM-51 mesoporous materials by means of 1HNMR spectroscopy, N2 adsorption, and HREM. a preliminary study. J Am Chem Soc 117:4049–4056
Nomura T, Zhu C, Sheng N, Tabuchi K, Sagara A, Akiyama T (2015) Shape-stabilized phase change composite by impregnation of octadecane into mesoporous SiO2. Sol Energy Mater Sol Cells 143:424–429
Yang Z, Zhao D, Xu M, Xu Y (1997) Analysis of the waterborne particles of bisphenol a type epoxy resin. Chem J Chin Univ 18:1568–1570
Zhu Y, Yang Z, Zhao D (2000) The mophological evolution of water droplets during incomplete phase inversion process. Acta Polym Sin 4:514–517
Acknowledgements
We would like to thank the Fund of Longshan academic talent research supporting program of SWUST (17LZXT04), and the Fund of Doctoral Program of SWUST (17zx7130).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fu, Z., Dai, L., Yi, Y. et al. Structure and thermal properties of stearic acid/silica composites as form-stable phase change materials. J Sol-Gel Sci Technol 87, 419–426 (2018). https://doi.org/10.1007/s10971-018-4752-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4752-5