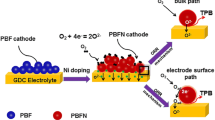
Abstract
Perovskite Ba0.8La0.2FeO3-δ was synthesized via a glycine–nitrate process as the cathode material for intermediate-temperature solid oxide fuel cells. Ultra-fine pure phase Ba0.8La0.2FeO3-δ powder was obtained after the self-propagating combustion of a complex xerogel precursor. The Ba0.8La0.2FeO3-δ cathode showed good compatibility and an enhanced phase interface with a samarium-doped ceria electrolyte. Electrochemical measurements showed that the Ba0.8La0.2FeO3-δ cathode achieved a low polarization resistance of 0.58 Ω cm2 at 800 °C in an air atmosphere. The porous nanostructure guaranteed the catalytic activity for the oxygen reduction reaction, even if the cathode material possessed a cobalt-free and low rare earth composition.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solid oxide fuel cells (SOFCs) have been recognized as a class of promising power-generating devices. A competitive advantage of SOFCs is their high energy conversion efficiency. Their efficiency can exceed 80% in the case of power-heat cogeneration [1], which is approximately equivalent to four times the efficiency of solar cells. However, the large-scale commercial application of SOFCs is restricted due to their high operating temperature, which is above 800 °C. It is thus urgent to develop intermediate-temperature solid oxide fuel cells (IT-SOFCs) that can operate in the temperature range of 600–800 °C [2, 3]. Despite their many practical merits, such as rapid start-up, high material reliability, and low running cost, IT-SOFCs suffer from increased electrolyte resistivity and electrode polarization. As far as the cathodes are concerned, cobalt-containing perovskite oxides exhibit a high electro-catalytic activity to the oxygen reduction reaction (ORR) below 800 °C. Unfortunately, the above-mentioned cathode materials that exhibit high thermal expansion coefficients (TECs) cannot satisfy the requirement of thermal compatibility with intermediate-temperature electrolyte materials. Therefore, the development of cobalt-free cathode materials has attracted more and more attention recently. For example, La0.8Sr0.2FeO3−δ [4], SrTi1−x Zr x O3 [5], SrFe1−x Ti x O3−δ [6], La0.5Sr0.5Fe0.9Mo0.1O3−δ [7], La0.6Sr0.4Fe0.8Cu0.2O3−δ [8] and La0.4Sr0.6Ni0.2Fe0.8O3−δ [9] have shown some exciting electrochemical or catalytic properties. In terms of price, the partial or entire substitution of A-site rare earths by alkaline earths can greatly reduce the costs of the cathode materials.
In this work, the Ba0.8La0.2FeO3-δ cathode material is synthesized through a glycine–nitrate route. The conversion of the complex xerogel precursor depends on a self-propagating combustion process to restrict the grain growth [10]. A non-stoichiometric ratio brings about changes in the electronic and ionic conductivities [11]. Chen et al. [12] prepared a Ba0.95La0.05FeO3-δ polycrystalline thin film with high ORR activity. When the molar ratio of the La3+ dopant is increased to 20%, more point defects, which influence the ORR catalytic activity, will be produced [13]. However, obvious lattice distortion occurs due to the high ratio of doping ions, which may result in a phase transition or ionic segregation. Therefore, effort should be devoted to suppressing the formation of impurities. Moreover, the porous nanostructure of the cathode for the electro-catalytic reaction should be tailored by controlling the sintering process [14–16]. Additionally, the ORR mechanism on the Ba0.8La0.2FeO3-δ cathode is investigated via electrochemical measurements.
2 Experimental
2.1 Preparation of Ba0.8La0.2FeO3-δ cathode material
Analytical grade Ba(NO3)2, La(NO3)3·6H2O, Fe(NO3)3· 9H2O, and C2H5NO2 (Sinopharm Chemical Reagent Co., Ltd, China) were used as the raw materials. The metal nitrates were dissolved in deionized water based on the chemical composition of Ba0.8La0.2FeO3-δ . According to the reaction shown in Eq. 1, the molar ratio of nitrates to glycine was kept at 1:2. The complexation between nitrates and glycine was conditioned at 70 °C with continuous stirring. Then, the solvent was gradually evaporated until initiating the self-propagating combustion of the xerogel. Subsequently, Ba0.8La0.2FeO3-δ materials were prepared by calcining the as-received precursor powder at 800–1000 °C for 2 h in a muffle furnace (Hefei Ke-Jing Materials Technology Co., Ltd, China). The cathode ink was prepared by mixing Ba0.8La0.2FeO3-δ powder with terpineol and ethyl cellulose, and then coated on the surface of the samarium-doped ceria (SDC , Sm0.2Ce0.8O1.9) electrolyte substrate by a screen-printing technique. Finally, the dried cathode layer was sintered at 1200 °C for 2 h. The electrolyte substrate using in this experiment was obtained by pressing the SDC ceramic powder ([17], referred to its preparation) under a pressure of 195 MPa and sintering at 1450 °C for 3 h.
2.2 Characterization of Ba0.8La0.2FeO3-δ cathode material
Thermal gravimetric-differential thermal analysis (TG-DTA) curves of the precursor powder that served as the cathode precursor were recorded using an STA409 thermogravimetric analyzer (Netzsch Corporation, Germany) from room temperature to 1200 °C at a heating rate of 5 °C min−1. The crystal phases of the as-received cathode materials were analyzed using a RINT2000 X-ray diffractometer (XRD, Rigaku Corporation, Japan) at a tube current of 30 mA and a tube voltage of 30 kV, with a scan rate of 5° min−1. Thermal expansion coefficient (TEC) measurement was performed in a push-rod dilatometer (Netzsch DIL 402 C, Germany) in air from room temperature to 800 °C at a heating rate of 2 °C min−1. The sample using in TEC measurement was prepared by molding Ba0.8La0.2FeO3-δ material into a cylinder and sintering it at 1200 °C for 2 h. The surface and cross-section morphologies of the cathode were observed using a Nova NanoSEM230 field emission scanning electron microscope (FE-SEM, FEI, Netherlands). Moreover, a durability test was performed by conditioning the cathode in 20 heating–cooling cycles: (1) Heat the sample from room temperature to 800 °C. (2) Hold the temperature at 800 °C for 2 h. (3) Cool the sample to room temperature.
Electrochemical measurements were carried out by a CHI 660B electrochemical workstation (Shanghai Chen-Hua Instruments, China) at 600–800 °C in ambient air. Sintered platinum films were used for both counter electrode and reference electrode. Linear sweep voltammetry was performed at a scan rate of 0.01 V s−1 in the cathode overpotential range of 0–0.5 V, and electrochemical impedance spectroscopy was performed at the open circuit potential with an AC disturbance of 0.005 V in the frequency range of 105–10−2 Hz.
3 Results and discussion
The TG-DTA curves of the precursor are shown in Fig. 1. The xerogel precursor consists of complex compounds which are formed by Ba2+, La3+, and Fe3+ (central ions) coordinated with glycine (ligand) and NO3 − (anion). As is well known, the precursor cannot completely transform to perovskite material during the rapid self-propagating combustion. The DTA curve initially shows an exothermic behavior around 200 °C, which is associated with the conversion of complex compounds to metal oxides. The heat of self-propagating reaction is not enough for the long range ordered atomic arrangement. Thus the interaction of generated metal oxides can be promoted due to their low grain sizes. The TG curve presents a tiny weight gain at approximately 400 °C, which is related to the combination reaction of BaO, Fe2O3, and O2. Then, the TG curve goes down slowly from 400 to 800 °C. The perovskite structure of BaFeO3 gradually generates in this temperature range, which results in an exothermic peak in the DTA curve at approximately 700 °C. Subsequently, a distinct weight loss caused by the formation of La-doped solid solution is observed from 800 to 950 °C. The stoichiometric ratio changes with the diffusion of La3+ into the BaFeO3 lattice. Simultaneously, the crystalline material loses oxygen atoms in the lattice to maintain the electroneutrality thus leading to the weight decrease. The calcination temperature of Ba0.8La0.2FeO3-δ cathode material can be determined according to the TG and DTA behaviors.
XRD patterns of the cathode materials prepared at different temperatures are presented in Fig. 2. Diffraction peaks corresponding to the (100), (110), (111), (200), (210), (220), and (310) crystal planes of cubic perovskite were detected. Clearly, a cathode material with high crystallinity can be obtained via a heat-treating step at 1000 °C, which is consistent with the results of the TG-DTA tests. According to the Debye-Scherrer equation [18], the average crystal size is close to 53 nm. In contrast, the crystallization process is accompanied by the diffusion of La3+ into Ba2+ lattice sites. When the heat-treating temperature is increased to 1000 °C, the impurity phases disappear due to the high ionic diffusion rate.
Figure 3 shows the cross-sectional morphology of the Ba0.8La0.2FeO3-δ cathode. As shown in Fig. 3a, there is no distinct crack at the cathode/electrolyte interface; i.e., good compatibility and low interfacial resistance between the porous cathode layer and dense electrolyte can be expected. Although the crystal size is not well-defined after the sintering of the nano-grains, a porous structure and many grain boundaries can be observed in Fig. 3b. Accordingly, nanostructured paths for electronic and ionic conduction as well as mass transport are constructed by controlling the crystallization and calcination processes. This cathode structure not only provides active sites for the electrochemical reduction of the oxygen but also promotes oxygen transport to the active sites. Figure 3c indicates that the grain size slightly grows and the porosity inevitably decreases after 20 heating–cooling cycles; however, the cathode layer is not peeled off the electrolyte due to the thermal mismatch. Linear thermal expansion curve of Ba0.8La0.2FeO3-δ material is presented in Fig. 4. The average TEC of Ba0.8La0.2FeO3-δ from 25 to 800 °C is 19.4 × 10−6 K−1. Compared with conventional cobalt-based cathode materials (20–30 × 10−6 K−1) [19], this value is close to that of SDC electrolyte (11.8 × 10−6 K−1) [8]. The better thermal compatibility is a definite advantage to prevent the crack formation during the heating–cooling cycles.
The iR-compensated cathode polarization curves of the Ba0.8La0.2FeO3-δ cathode at 600–800 °C in air are illustrated in Fig. 5. Compared with previously reported cobalt-free cathode materials, Ba0.8La0.2FeO3-δ exhibits a relatively low polarization loss, as shown in Table 1. It is well known that the oxygen partial pressure (p O2) in an air atmosphere is only 0.21 × 105 Pa. Therefore, the cathode performance will improve further if pure oxygen is used as the oxidant. At the same working current, the cathode overpotential is reduced with the increasing operation temperature. The contribution of the temperature is associated with the faster conduction of charge carriers and higher activity of oxygen molecules [20]. The temperature-dependent mechanism of the Ba0.8La0.2FeO3-δ material towards ORR can be summarized as follows: (1) Introduction of La3+ generates excessive positive charges. (2) Excessive charges are compensated by changing the valence of Fe4+ to Fe3+ and generating oxygen vacancies. (3) The high mobility of O2− in the high temperature region promotes the incorporation of oxygen atoms (reactant) into oxygen vacancies.
Nyquist diagrams of the Ba0.8La0.2FeO3-δ cathode at 600–800 °C in air are shown in Fig. 6. The capacitance arcs in the high- and low-frequency regions are associated with the oxygen adsorption and reduction processes, respectively. The inset in Fig. 5 presents the fitting equivalent circuit for the impedance spectrums, among which R O denotes the ohmic resistance between the cathode and the reference electrode, R 1 represents the resistance of the O2 dissociative adsorption on the cathode surface, and R 2 represents the charge transfer resistance of Oads to O2−. L is the inductance caused by the response relaxation to the high frequency, and Q 1 and Q 2 are the constant phase elements associated with the double-layer capacitance. Table 2 presents the values of the impedance elements fitted by ZsimpWin software. The polarization resistance (R p), which characterizes the electrocatalytic activity, can be determined by calculating the sum of R 1 and R 2. As shown in Table 2, the values of R 2 are higher than the corresponding values of R 1, which indicates that the charge transfer step dominates the electrochemical kinetic processes. The polarization resistance of the Ba0.8La0.2FeO3-δ cathode is 0.58 Ω cm2 at 800 °C in air conditions. This value is slightly higher than those of cobalt-containing cathode materials reported in the recent literature, e.g., Ba2Bi0.1Sc0.2Co1.7O6-x (0.22 Ω cm2) [21] and Pr0.3Sr0.7Co0.3Fe0.7O3−δ (0.174Ω cm2) [22], but is comparable to that of cobalt-free Ba0.95Ca0.05FeO3-δ (0.60 Ω cm2) [23]. Furthermore, good compatibility between the cathode and the SDC electrolyte guarantees the long-term structural stability of the fuel cell. In addition, the lower content of rare earth in contrast to traditional (La, Pr)0.8Sr0.2FeO3-δ cathodes [4, 24] is of great significance to reduce the operating cost. The deficiency in the polarization resistance will be improved by optimizing the doping cations (e.g., Sm, Ce, Pr) and cathode structure in future work.
4 Conclusions
Perovskite Ba0.8La0.2FeO3-δ cathode material for IT-SOFCs was synthesized via a glycine–nitrate process. Compared to traditional cathodes with high contents of cobalt and rare earth, the Ba0.8La0.2FeO3-δ cathode possessed a higher polarization resistance. Nevertheless, an acceptable electrocatalytic activity was obtained in the intermediate-temperature region, which benefited from the porous nanostructure. Moreover, good compatibility between the Ba0.8La0.2FeO3-δ cathode and Sm0.2Ce0.8O1.9 electrolyte guaranteed the long-term structural stability. Therefore, the Ba0.8La0.2FeO3-δ material can be considered a promising cathode candidate for IT-SOFCs.
References
Shaikh SPS, Muchtar A, Somalu MR (2015) A review on the selection of anode materials for solid-oxide fuel cells. Renew Sust Energ Rev 51:1–8
Li P, Zhao YC, Yu BL, Li J, Li YD (2015) Improve electrical conductivity of reduced La2Ni0.9Fe0.1O4+δ as the anode of a solid oxide fuel cell by carbon deposition. Int J Hydrog Energy 40:9783–9789
Samat AA, Somalu MR, Muchtar A, Hassan OH, Osman N (2016) LSC cathode prepared by polymeric complexation method for proton-conducting SOFC application. J Sol Gel Technol 78:382–393
Simner SP, Bonnett JF, Canfield NL, Meinhardt KD, Shelton JP, Sprenkle VL, Stevenson JW (2003) Development of lanthanum ferrite SOFC cathodes. J Power Sources 113:1–10
Xiao J, Han D, Yu FY, Zhang L, Liu J, Zhan ZL, Zhang YJ, Dong P (2016) Characterization of symmetrical SrFe0.75Mo0.25O3-δ electrodes indirect carbon solid oxide fuel cells. J Alloy Compd 688:939–945
Yu XL, Long W, Jin FJ, He TM (2014) Cobalt-free perovskite cathode materials SrFe1-x Ti x O3-δ and performance optimization for intermediate-temperature solid oxide fuel cells. Electrochim Acta 123:426–434
Wang SL, Feng Y, Wang DS (2014) Electrochemical comparison of cobalt-free La0.5Sr0.5Fe0.9Mo0.1O3-δ based cathode materials for intermediate-temperature solid oxide fuel cells. Ceram Int 40:6359–6363
Zhou QJ, Xu L, Guo YJ, Jia D, Li Y, Wei WCJ (2012) La0.6Sr0.4Fe0.8Cu0.2O3-δ perovskite oxide as cathode for IT-SOFC. Int J Hydrog Energy 37:11963–11968
Zhu GY, Fang XH, Xia CR, Liu XQ (2005) Preparation and electrical properties of La0.4Sr0.6Ni0.2Fe0.8O3 using a glycine nitrate process. Ceram Int 31:115–119
Guo XZ, Zhang QL, Ding XG, Shen QH, Wu CC, Zhang LJ, Yang H (2016) Synthesis and application of several sol–gel-derived materials via sol–gel process combining with other technologies: a review. J Sol Gel Technol 79:328–358
Roldán-Carmona C, Gratia P, Zimmermann I, Grancini G, Gao P, Graetzel M, Nazeeruddin MK (2015) High efficiency methylammonium lead triiodide perovskite solar cells: the relevance of non-stoichiometric precursors. Energy Environ Sci 8:3550–3556
Chen DJ, Chen C, Dong FF, Shao ZP, Ciucci F (2014) Cobalt-free polycrystalline Ba0.95La0.05FeO3-δ thin films as cathodes for intermediate-temperature solid oxide fuel cells. J Power Sources 250:188–195
Mahato N, Banerjee A, Gupta A, Omar S, Balani K (2015) Progress in material selection for solid oxide fuel cell technology: a review. Prog Mater Sci 72:141–337
Long T, Cui XY, Li L, Li SM, Xu LY (2015) Synthesis of hierarchically porous ZrO2 monolith by a template method. Mater Manuf Process 30:571–575
Oh EO, Whang CM, Lee YR, Park SY, Prasad DH, Yoon KJ, Son JW, Lee JH, Lee HW (2012) Extremely thin bilayer electrolyte for solid oxide fuel cells (SOFCs) fabricated by chemical solution deposition (CSD). Adv Mater 24:3373–3377
Karpinsky DV, Troyanchuk IO, Bushinsky MV, Gavrilov SA, Silibin MV, Franz A (2016) Crystal structure and magnetic properties of Bi1−x Ca x Fe1−x Mn(Ti) x O3 ceramics across the phase boundary. J Mater Sci 51:10506–10514
Mori T, Buchanan R, Ou DR, Ye F, Kobayashi T, Kim JD, Zou J, Drennan J (2008) Design of nanostructured ceria-based solid electrolytes for development of IT-SOFC. J Solid State Electrochem 12:841–849
Pereira FJ, Díez MT, Aller AJ (2015) Effect of temperature on the crystallinity, size and fluorescent properties of zirconia-based nanoparticles. Mater Chem Phys 152:135–146
Lee KT, Manthiram A (2006) Comparison of Ln0.6Sr0.4CoO3-δ (Ln=La, Pr, Nd, Sm, and Gd) as cathode materials for intermediate temperature solid oxide fuel cells. J Electrochem Soc 153:A794–A798
Navarrete L, Solís C, Serra JM (2015) Boosting the oxygen reduction reaction mechanisms in IT-SOFC cathodes by catalytic functionalization. J Mater Chem A 3:16440–16444
Zhou W, Sunarso J, Chen ZG, Ge L, Motuzas J, Zou J, Wang GX, Julbe A, Zhu ZH (2011) Novel B-site ordered double perovskite Ba2Bi0.1Sc0.2Co1.7O6-x for highly efficient oxygen reduction reaction. Energy Environ Sci 4:872–875
Park K, Kim J, Bae J (2015) Electrochemical analysis of Pr0.3Sr0.7Co x B(1−x)O3-δ (B = Fe, Mn; x = 0, 0.3, 0.5, 0.7, and 1) as cathode materials for intermediate temperature SOFCs. Solid State Ionics 272:45–52
Wang J, Lam KY, Saccoccio M, Gao Y, Chen DJ, Ciucci F (2016) Ca and In co-doped BaFeO3-δ as a cobalt-free cathode material for intermediate-temperature solid oxide fuel cells. J Power Sources 324:224–232
Chen YH, Gu QW, Tian D, Ding YZ, Lu XY, Yu WL, Isimjan TT, Lin B (2014) (La, Pr)0.8Sr0.2FeO3-δ –Sm0.2Ce0.8O2-δ composite cathode for proton-conducting solid oxide fuel cells. Int J Hydrog Energy 39:13665–13670
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, P., Zhu, H., Kong, J. et al. Preparation and electrochemical properties of Ba0.8La0.2FeO3-δ cathode for intermediate-temperature solid oxide fuel cells. J Sol-Gel Sci Technol 82, 233–238 (2017). https://doi.org/10.1007/s10971-016-4300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4300-0