Abstract
In this work, a new porous solid polysiloxane material with thiol-amine-based multiple functional groups polysiloxane-(monoamine-thiol) triacetate, P-(N-S)-TrEA (where P- represents [Si–O]n polysiloxane network), was synthesized using one-pot reaction of TEOS with 3-iminodiethylacetatepropyltrimethoxysilane and 3-thiolethylacetatepropyltrimethoxysilane in the presence of CTAB as a surfactant. Its ethylenediamine and diethylenetriamine-modified polysiloxane-(monoamine-thiol)-tris(N-2-aminoethylacetamide), P-(N-S)-TrEA-NN, and polysiloxane-(monoamine-thiol)-tris(N-3,6-diazahexylacetamide), P-(N-S)-TrEA-NNN, chelating ligand systems were also obtained. The previously reported simple functionalized polysiloxane (monoamine-thiol) has been modified to obtain thiol-monoamine-based multiple functional groups. A variety of methods and techniques including FTIR, solid-state 13C NMR, XPS and TGA have been used for establishing their structure.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Silica-based solid supports have gained much importance over other supports in the last years because of their ability of withstand chemical and heat treatments, high pressure degradation, straining and swelling [1–5]. In addition, chelating agents can be easily bound chemically to these supports, affording high stability [6]. The incorporation of chelating groups onto inorganic and hybrid silica-based solid supports exhibits increasing number of applications in areas such as extraction, recovery and separation of metal ions from aqueous solutions as well as chromatographic stationary phases and heterogeneous catalysis [7–20].
Silica-based functionalized polysiloxanes have been prepared either by the low-temperature sol–gel process or by modification of pre-prepared polysiloxanes [7–20]. Many mono-functionalized polysiloxanes have been prepared in the last few decades including amines, thiols, iminodiacetic acids, amino acids and macrocyclic [21].
Some simple bi-functionalized polysiloxanes including thiol–amine [22], phosphine–monoamine, phosphine–diamine and phosphine–thiol ligand systems were prepared by hydrolytic polycondensation of mixtures of TEOS and two different silane precursors [23]. In these systems, two types of ligand groups were introduced into the polysiloxane matrix during the sol–gel process [22, 23]. The sol–gel process permits the preparation of relatively homogeneous immobilized-polysiloxane ligand systems containing two types of ligand groups with specific molar ratios, on the same polysiloxane framework [22, 23].
The bi-functionalized immobilized-polysiloxane ligand systems give wide range and high uptake capacity toward different types of metal ions from aquatic media [20]. Further modification of the known bi-ligand systems is not yet investigated. In this paper, the monoamine and thiol-silane precursors were modified by functionalization with ethyl acetate groups and then mixed with TEOS precursor via template sol–gel method to give polysiloxane-(monoamine-thiol) triethylacetate. Further modification of the triethylacetate (monoamine-thiol) ligand system was made in order to obtain multiple functionalized polysiloxane-(monoamine-thiol)-tris(N-2-aminoethylacetamide) and polysiloxane-(monoamine-thiol)-tris(N-3,6-diazahexylacetamide).
2 Experimental
2.1 Reagents and materials
Tetraethylorthosilicate, 3-aminopropyltrimethoxysilane, 3-mercaptopropyltrimethoxysilane and ethylchloroacetate were purchased from MERCK and used as received. Cetyltrimethylammonium bromide (CTAB) surfactant was purchased from MERCK and used as received. Ethylenediamine, diethylenetriamine, absolute ethanol, toluene, diethyl ether, acetone and absolute methanol were purchased from SIGMA Aldrich Company and used as received without further purification.
2.2 Characterization of the materials
13C CP-MAS solid-state NMR experiments were carried out at room temperature on Bruker MSL-400 MHz spectrometer at frequency of 100.6 MHz (13C) using a Bruker CP/MAS probe. Proton decoupling was always applied during acquisition. Solid samples were spun at 5 kHz using 7-mm ZrO2 rotors filled in a glove box under dried argon atmosphere.
The X-ray photoelectron spectra (XPS) were recorded on an ESCALAB 250, spectrometer equipped with a monochromatic AlKα X-ray source (hν = 1486.6 eV, 650 μm spot size). An electron flood gun was used to obtain a perfectly uniform static charge over the sample area analyzed. The filament current was 3 A, and the emission current 0.2 mA. These conditions yield negative but uniform static charge over the powder surface. Binding energy positions were calibrated against the main C–C/C–H C1s component set at 285 eV. The surface elemental composition was determined by considering the peak areas and the corresponding Scofield sensitivity factors corrected for the electron analyzer transformation function.
Thermogravimetric analysis TGA was carried out using Mettler Toledo SW 7.01 analyzer in the range of 25–600 °C under nitrogen.
Elemental analysis for carbon, hydrogen and nitrogen was carried out, using an Elemental Analyzer EA 1110-CHNS CE Instrument.
The infrared spectra of the materials were recorded on a Perkin-Elmer FTIR, spectrometer using KBr disk in the range of 4000–400 cm−1.
The surface morphology of polysiloxane-(monoamine-thiol) triethylacetate ligand system was characterized by using Optica-B-350 microscope.
2.3 Preparations
2.3.1 Preparation of mesoporous immobilized-polysiloxane (monoamine-thiol) triethylacetate polysiloxane (P-(N-S)-TrEA)
This material was prepared by sol–gel process in three steps, as reported previously [22, 23]:
-
(1)
Preparation of 3-thiolethylacetatepropyltrimethoxysilane, (MeO)3Si(CH2)3-SCH2CO2Et, (L-SA).
Ethylchloroacetate (10.0 g, 0.08 mol) was added dropwise (2 g/min) to a stirred solution of 3-mercaptopropyltrimethoxysilane (6.13 g, 0.05 mol) in 15 mL absolute ethanol. The mixture was refluxed at 100 °C for 12 h. Then, the mixture was cooled at room temperature.
-
(2)
Preparation of 3-iminodiethylacetatepropyltrimethoxysilane, (MeO)3Si(CH2)3N(CH2CO2Et)2, (L-IDA).
Ethylchloroacetate (15.0 g, 0.122 mol) was added dropwise (2 g/min) to a stirred solution of 3-aminopropyltrimethoxysilane (9.80 g, 0.05 mol) in 30 mL absolute ethanol. The mixture was refluxed at 100 °C for 12 h. Then, the mixture was cooled at room temperature.
The modified silane precursors L-SA and L-IDA were kept in solution at room temperature.
-
(3)
Sol–gel step.
Solutions of the modified silane precursors L-SA and L-IDA were added to a stirred solution of tetraethylorthosilicate (20.8 g, 0.10 mol), in the presence of cetyltrimethylammonium bromide (CTAB) (3.64 g) in absolute ethanol (30 mL) and hydrochloric acid solution (4.95 g, 0.42 M) as a catalyst. Gelation occurred within 3 days. The product was aged for another 3 days and then dried in a vacuum oven at 80 °C for 12 h. The solid material (P-(N-S)-TrEA) was crushed and sieved to a particle size range of (125–150) μm. Cetyltrimethylammonium bromide (CTAB) was removed by refluxing with hot ethanol. The material was then washed successively with 50 mL portions of 0.025 M NaOH, water, methanol and diethylether. The solid material was dried in a vacuum oven at 80 °C for 12 h.
2.3.2 Preparation of immobilized-polysiloxane (monoamine-thiol) triacetic acid (P-(N-S)-TrAA)
The immobilized-polysiloxane (monoamine-thiol) triethylacetate P-(N-S)-TrEA was hydrolyzed by refluxing (5.0 g) of the ester material with 150 mL of 2.0 M HCl for 6 h. The acid form of polysiloxane triacetic acid ligand system P-(N-S)-TrAA was then filtered, washed successively with 50 mL portions of 0.025 M NaOH, water, methanol and diethylether and then dried in a vacuum oven at 80 °C for 12 h.
2.3.3 Preparation of immobilized-polysiloxane (monoamine-thiol) tris(N-2-aminoethylacetamide) (P-(N-S)-TrEA-NN)
The immobilized-polysiloxane (thiol-monoamine)-triethylacetate P-(N-S)-TrEA (5.0 g) was refluxed with ethylenediamine (10 g, 0.17 mol) in 50 mL anhydrous toluene with stirring at 110 °C for 48 h. Then, the mixture was cooled, filtered off and washed with ethanol. The product P-(N-S)-TrEA-NN was then dried in a vacuum oven at 80 °C for 12 h.
2.3.4 Preparation of immobilized-polysiloxane (monoamine-thiol)-tris(N-3,6-diazahexylacetamide), P-(N-S)-TrEA-NNN
The immobilized-polysiloxane (monoamine-thiol) triethylacetate P-(N-S)-TrEA (5.0 g) was refluxed with diethylenetriamine (17.0 g, 0.16 mol) in 50 mL anhydrous toluene with stirring at 110 °C for 48 h. Then, the mixture was cooled, filtered off and washed with ethanol. The product P-(N-S)-TrEA-NNN was dried in a vacuum oven at 80 °C for 12 h.
3 Results and discussion
3.1 Synthesis
The functionalized immobilized-polysiloxane (monoamine-thiol) triacetic acid, P-(N-S)-TrAA, (monoamine-thiol) tris(N-2-aminoethylacetamide), P-(N-S)-TrEA-NN, and monoamine-thiol-tris(N-3.6-diazahexylacetamide, P-(N-S)-TrEA-NNN, were prepared using template procedure in the presence of CTAB by the following steps (Schemes 1, 2, 3, 4, 5). The elemental analysis is given in Table 1.
-
(1)
Preparation of 3-iminodiethylacetatepropyltrimethoxysilane and 3-thiolethylacetatepropyltrimethoxysilane by treatment the 3-aminopropyltrimethoxysilane and 3-mercaptopropyltrimethoxysilane with ethylchloroacetate in absolute ethanol (Scheme 1).
-
(2)
The two silane agents were mixed with TEOS in the presence of CTAB surfactant. CTAB was then removed by reflux with ethanol to obtain mesoporous (monoamine-thiol) triethylacetate polysiloxane ligand system, P-(N-S)-TrEA (Scheme 2).
-
(3)
Formation of immobilized-polysiloxane (monoamine-thiol) triacetic acid, P-(N-S)-TrAA was obtained by hydrolysis of P-(N-S)-TrEA ester precursor using 2 M HCl (Scheme 3).
-
(4)
Preparation of immobilized-polysiloxane P-(N-S)-TrEA-NN by the reaction of P-(N-S)-TrEA with ethylenediamine (Scheme 4).
-
(5)
Preparation of immobilized-polysiloxane P-(N-S)-TrEA-NNN by the reaction of P-(N-S)-TrEA with diethylenetriamine (Scheme 5).
3.2 Elemental analysis
From the elemental analysis given in Table 1, it is clear that carbon percentage decreases from 17.75 to 12.81 % and the nitrogen percentage decreases slightly from 2.55 to 2.43 % after hydrolysis of P-(N-S)-TrEA by 2 M HCl.
Upon treatment of P-(N-S)-TrEA material with ethylenediamine or diethylenetriamine groups, it is observed that there is an increase in the found nitrogen percentage from 2.55 to 7.52 % for P-(N-S)-TrEA-NN and from 2.55 to 7.74 % for P-(N-S)-TrEA-NNN (Table 1). There is also an increase in carbon percentages from 17.75 to 21.82 % for P-(N-S)-TrEA-NN and from 17.75 to 23.40 % for P-(N-S)-TrEA-NNN (Table 1). This provides evidence that ethylenediamine and diethylenetriamine groups have been introduced into P-(N-S)-TrEA material.
It is observed that the found values are lower than the expected elemental analysis values in the case of carbon for P-(N-S)-TrEA and its acid form, which is probably attributed to the incomplete hydrolysis of alkoxy groups [19–24].
The slight increase of sulfur percentage is due to the complete attachment of functionalized thiol-silane agent in comparison with the functionalized monoamine silane agent based on the actual molecular weight of the material. That is also evident from the relative intensities of 13C NMR peaks of methylene carbons attached to N or S atoms. This behavior is probably due to the difference in the rate of hydrolysis and polycondensation between the two silane agents. Therefore, the incorporation of the sulfur-containing functional group onto the siloxane support is much more than that of the amine functional group.
3.3 FTIR spectra
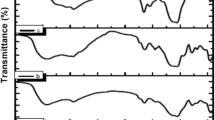
FTIR spectra for the immobilized-polysiloxane ligand systems: P-(N-S)-TrEA/CTAB, P-(N-S)-TrEA, P-(N-S)-TrAA, P-(N-S)-TrEA-NN and P-(N-S)-TrEA-NNN, are given in Fig. 1a–e. The spectra show three regions of absorption bands at 3500–3000 cm−1 due to ν(O–H) or ν(N–H), 1750–1500 cm−1 due to δ(O–H), δ(N–H) or ν(C=O) and 1200–900 cm−1 due to ν(Si–O) [23–25]. Figure 1a, b shows a characteristic band at 2560 cm−1 due to ν(S–H) which is due to thiol functionalized ligand. The bands around 2980 cm−1 are due to symmetric and asymmetric ν(C–H) aliphatic stretching vibrations. These absorption bands confirm that both monoamine and thiol functional groups are covalently bound to polysiloxane matrix. The FTIR spectrum for the immobilized-polysiloxane P-(N-S)-TrEA/CTAB ligand system (Fig. 1a) shows a band at 2940 cm−1 which was disappeared in the immobilized P-(N-S)-TrEA ligand system (Fig. 1b) due to the removal of CTAB surfactant [19, 20]. Figure 1a shows a strong band at 1740 cm−1 due to ν(C=O) vibration of the ester form (–COOEt), whereas the spectrum of the immobilized-polysiloxane P-(N-S)-TrAA acid form (Fig. 1c) shows a strong absorption band at 1670 cm−1 due to ν(C=O) vibration. This indicates that all ester groups (–CH2COOEt) were converted into the acid form (–CH2COOH) [19, 20]. The absorption spectra of the immobilized-polysiloxane P-(N-S)-TrEA-NN and P-(N-S)-TrEA-NNN ligand systems (Fig. 1d, e) show a characteristic absorption band at 1658 cm−1 for the ν(N–C=O) due to the amide stretching vibration. The spectra show two peaks at 3092 cm−1 ν(N–H) and 3361 cm−1 that assigned for the ν(N–H) symmetric and asymmetric stretching vibrations. The spectra also show a strong band at 1549 cm−1 due to δ(N–H) bending vibrations. The absence of ν(C=O) band at 1760 cm−1 of the ethylchloroacetate after the reaction with the ethylenediamine and diethylenetriamine is a strong evidence of the complete conversion of the acetate groups into the amine groups [26]. These FTIR assignments were based on reported spectral data of similar materials [18–26]. Further confirmation for these changes was obtained from XPS and NMR results discussed later.
3.4 13C NMR spectra
The CP/MAS 13C spectra for P-(N-S)-TrEA and P-(N-S)-TrAA are given in Fig. 2a, b. The spectrum for the immobilized-polysiloxane (monoamine-thiol) triethylacetate ligand system P-(N-S)–TrEA (Fig. 2a) shows signals at 11, 22 and 43 ppm corresponding to C1/C*1, C2 and C3 (Scheme 6). The signal at 36 ppm is due to C*2 and C*3. The shoulder signals at 51 ppm are due to C4/C*4 [25, 26]. The broad signal at 175 ppm is due to carbonyl C*5 and C5. The two signals at 63 and 15 ppm correspond to C6 and C7 of ethyl acetate group. The spectrum of P-(N-S)-TrAA (Fig. 2b) shows similar signals as that of P-(N-S)-TrEA, with some changes. The signals at 15 and 63 ppm were disappeared which provide strong evidence that the ester form was completely changed to the acid form. The signal at 178 ppm (s) is probably due to carbonyl group of the acid form (Scheme 7) which slightly shifted to more down field. All these assignments are based on reported spectral data of similar systems [22–26].
The 13C CP NMR spectrum for the immobilized-polysiloxane ligand systems P-(N-S)-TrEA-NN and P-(N-S)-TrEA-NNN (Schemes 8 and 9) is shown in Fig. 3. The spectra show signals at 12 and 24 ppm that correspond to C*1/C1 and C2 methylene carbon atoms. The broad strong signal centered at 40 ppm is probably composed of three peaks nearly at 36, 43 and 50 ppm due to C*2/C*3, C6/C7 and C4/C*4, respectively. The spectrum shows two carbonyl carbons at 164 and 173 ppm due to absorbed CO2 and C5/C*5, respectively (Schemes 8, 9) [22–26]. No signals were observed due to OCH2CH3 species which give an evidence for the introduction of ethylenediamine and diethylenetriamine onto P-(N-S)-TrEA material.
3.5 XPS results
The XPS survey spectra for the immobilized-polysiloxane ligand systems P-(N-S)-TrEA/CTAB, P-(N-S)-TrEA, P-(N-S)-TrAA, P-(N-S)-TrEA-NN and P-(N-S)-TrEA-NNN are shown in Fig. 4a–e. The spectra show main peaks at 102, 164, 285, 400 and 532 eV corresponding to Si2p, S2p, C1s, N1s and O1s. The atomic percentages of carbon, silicon, oxygen, nitrogen and their corresponding core-line binding energies (BEs) are given in Table 2. The XPS survey spectrum of the immobilized-polysiloxane P-(N-S)-TrEA/CTAB ligand system (Fig. 4a) shows high intensity of carbon percentage due to the presence of CTAB which decreases after removal of CTAB (Fig. 4b). This is also confirmed from the atomic percentage of carbon which decreases from 40.14 to 35.05 % after removal of CTAB (Table 2). When the P-(N-S)-TrEA material is treated with ethylenediamine or diethylenetriamine, a significant increase of nitrogen and carbon percentages was observed (Fig. 4d, e; Table 2). This indicates the introduction of ethylenediamine and diethylenetriamine groups into the P-(N-S)-TrEA material.
The reason why bulk elemental analysis for carbon percentage for P-(N-S)-TrEA (Table 1) is different from that obtained from XPS (Table 2) is that there has non-uniform distribution of functional groups from the top surface to deep inside the particle, but probably there has uniform distribution of functional groups only on the surface of the particles. Most of functional groups are onto the particle surface, and probably few are trapped inside the bulk.
All these assignments were based on reported data of similar systems [20, 26]. Figures 5, 6, 7, 8 and 9 show the fitted C1s carbon of P-(N-S)-TrEA/CTAB, P-(N-S)-TrEA, P-(N-S)-TrAA, P-(N-S)-TrEA-NN and P-(N-S)-TrEA-NNN, which indicated the presence of three signals centered at 284.99, 286.54 and 289.04 eV corresponding to three C1s components (C–C/C–H, C–N/C–O and COOH/COOR) (Table 3). These results are similar to the reported data [20, 26].
These indications could be concluded from the data given in Table 3 as follows:
-
The removal of CTAB has been reflected by decreasing of the C1s (B) component from 18.8 to 16.5.
-
It is notable that upon hydrolysis of P-(N-S)-TrEA to acid form P-(N-S)-TrAA, the C1 s (B) component has slightly decreased from 16.5 to 13.9 % due to change of –COOR to acid form –COOH.
-
The introduction of amine groups was confirmed by increasing C1s (B) component from 16.5 to 25.76 % for ethylenediamine and to 20.93 % for diethylenetriamine.
The N1s fitted curves for P-(N-S)-TrEA/CTAB, P-(N-S)-TrEA, P-(N-S)-TrAA, P-(N-S)-TrEA-NN and P-(N-S)-TrEA-NNN are shown in Figs. 10, 11, 12, 13 and 14, and percentages of N1s (A), N1s (B) and N1s (C) components are given in Table 4 for the free nitrogen, hydrogen bonding and protonated amine, respectively. All the three N1s (A), N1s (B) and N1s (C) components of N1s for P-(N-S)-TrEA/CTAB are shown in Fig. 10. In the case of the system P-(N-S)-TrEA (Fig. 11), only two components centered at 399.67 and 401.76 eV assigned to the free and hydrogen bonding amine, respectively. The protonated amine was not observed due to the removal of CTAB.
Upon hydrolysis of P-(N-S)-TrEA by 2 M HCl (Fig. 12; Table 4), N1s fitting spectrum of P-(N-S)-TrAA displays two components centered at 399.69 and 401.76 eV. These are assigned to the free amine and hydrogen bonding amine, respectively.
The N1s spectra of P-(N-S)-TrEA-NN and P-(N-S)-TrEA-NNN are fitted with two components centered at 399.96, 401.70 and 399.77, 401.57 eV, respectively, which assigned for the free nitrogen and the hydrogen bonding amine, respectively (Figs. 13, 14). There are a significant increase of free N1 s component and a decrease of hydrogen bonding component upon treatment of P-(N-S)-TrEA material with ethylenediamine or diethylenetriamine groups (Table 4). This is confirmed by the increases in nitrogen percentage from 0.55 to 4.95 and 0.55 to 6.00, respectively (Table 2).
The molar ratio (N/Si) increases from 1:17 to 1:1 by the treatment of P-(N-S)-TrEA material with ethylenediamine and diethylenetriamine (Table 2), which gives a strong evidence that the ethylenediamine and diethylenetriamine groups are introduced into the P-(N-S)-TrEA material.
3.6 Thermal analysis
Thermogravimetric analysis (TGA) and differential thermogravimetric analysis (DTA) were examined for P-(N-S)-TrEA, P-(N-S)-TrAA, P-(N-S)-TrEA-NN and P-(N-S)-TrEA-NNN under nitrogen at temperature range of 25–600 °C with 10 °C/min. The weight loss of the polysiloxanes was studied as a function of change in temperature. Figure 15 shows the thermograms of P-(N-S)-TrEA-immobilized ligand system. Four DTA peaks (maxima) were observed. This can be explained by a four-stage degradation process for the P-(N-S)-TrEA. The first peak at ~65.8 °C is attributed to the loss of physisorbed water (dehydration) and alcohol from the pore system [27–29]. The second peak at ~264.2 °C is due to dehydroxylation of surface of OH silanol groups. The third stage at ~392.5 °C is probably due to cleavage and degradation of the organofunctional group bonds which attached directly to the silicon atoms producing gases, e.g., CO2, and formaldehyde (H2CO) which takes place as a product of the thermal decomposition [30–32]. The DTA curve after 400 °C and up to 600 °C is attributed to further dehydroxylation of surface silanol groups, forming siloxane bonds, [30–33]. The loss of weight for P-(N-S)-TrEA is 35.6 %, which decreases to 33.5 % after hydrolysis of P-(N-S)-TrEA (Fig. 16).
When P-(N-S)-TrEA is treated with ethylenediamine and diethylenetriamine, the DTA thermogram for P-(N-S)-TrEA-NN shows five maxima and six maxima for P-(N-S)-TrEA-NNN as shown in Figs. 17 and 18, respectively. The loss of weight increases from 35.6 % for P-(N-S)TrEA to 36.0 % for P-(N-S)-TrEA-NN and to 42.1 % for P-(N-S)-TrEA-NNN. The extra peaks are probably due to degradation of ethylenediamine and diethylenetriamine organic groups (Figs. 17, 18). The extra peak at 235 °C is probably due to carbon dioxide CO2 captured by ethylenediamine and diethylenetriamine functionalized silica [33]. These peaks are not shown in its parent P-(N-S)TrEA, which confirmed that their appearance refers the presence of amine groups as reported [33].
3.7 Polarized optical microscopy
Photographs was taken using polarized optical microscope for P-(N-S)-TrEA/CTAB (Fig. 19). It shows a spherical shape with an average particle size and pore size of 12.06 and 3.09 μm, respectively, with hexagonal pore. It can be seen that all particles are of the same shape with well-defined boundaries. The fine features of the particles are well clear and visible.
4 Conclusion
The immobilized-polysiloxane monoamine-thiol triacetic acid P-(N-S)-TrAA was prepared by sol–gel method. The ethylenediamine, P-(N-S)-TrEA-NN and diethylenetriamine P-(N-S)-TrEA-NNN derivatives were prepared by modification of P-(N-S)-TrEA ester form. These materials were well investigated by several techniques including FTIR, 13C CP NMR, XPS, TGA, polarized optical microscope and elemental analysis. These techniques confirmed that both monoamine and thiol-silane coupling agents were not equally incorporated onto siloxane matrix through the sol–gel process. The thermogravimetric analysis provided evidence that these materials are thermally stable at elevated temperatures.
References
McLaren JW, Mykytiuk AP, Willie SN, Berman SS (1985) Anal Chem 57:2907–2911
Sharma RK (2001) Pure Appl Chem 73(1):181–186
Kvitek RJ, Evans JF, Carr PW (1982) Anal Chim Acta 144:93–98
Van Grieken R (1982) Anal Chim Acta 143:3–34
Sulthivaiyakit P, Kettrup A (1985) Anal Chim Acta 169:331–339
Jal PK, Dutta RK, Sudershan M, Saha A, Bhattacharya SN, Chintalapudi SN, Mishra BK (2001) Talanta 55:233–240
Khatib IS, Parish RV (1989) J Organomet Chem 369:9–16
El-Nahhal IM, Parish RV (1993) J Organomet Chem 452:19–22
Ahmed I, Parish RV (1993) J Organomet Chem 452:23–28
El-Nahhal IM, El-Shetary BA, Salib KAR, El-Ashgar NM, El-Hashash AM (2001) Anal Lett 34:2189–2202
El-Nahhal IM, El-Ashgar NM, Chehimi MM (2003) Microporous Mesoporous Mater 65(2–3):299–310
El-Ashgar NM (2008) J Chem 5:107–113
Saadeh SM, El-Ashgar NM, El-Nahhal IM, Chehimi MM, Maquet J, Babonneau F (2005) Appl Organomet Chem 19:759–767
El-Ashgar NM, El-Nahhal IM, Chehimi MM, Babonneau F, Livage J (2005) Monats Chem 137:263–275
El-Nahhal IM, El-Ashgar NM, Livage J, Chehimi MM, Babonneau F (2007) Mater Lett 61:4553–4558
El-Ashgar NM, El-Nahhal IM, Chehimi MM, Babonneau F, Livage J (2009) Int J Environ Anal Chem 89(14):1057–1069
Innocenzi P, Zub YL, Kessler VG (2008) Sol–gel methods for materials processing, NATO science for peace and security series C: environmental security. Springer, New York
El-Ashgar NM, El-Nahhal IM, Chehimi MM, Babonneau F, Livage J (2012) Phosphorus Sulfur Silicon Relat Elem 187:392–402
El-Nahhal IM, El-Ashgar NM, Abu-Shawish AA, Ahmed MA, Babonneau F (2015) Phosphorus Sulfur Silicon Relat Elem. doi:10.1080/10426507.2015.1012667
El-Ashgar NM, Silmi MK, El-Nahhal IM, Chehim MM, Babonneau F (2015) Silicon. doi:10.1007/s12633-015-9303-x
El-Nahhal IM, El-Ashgar NM (2007) J Organomet Chem 692:2861–2886
El-Nahhal IM, Yang JJ, Chuang IS, Maciel GE (1996) J Non Cryst Solids 208:105–117
Yang JJ, El-Nahhal IM, Maciel GE (1997) J Non Cryst Solids 212:281–291
El-Nahhal IM (2000) Phosphorus Sulfur Silicon Relat Elem 162:245–258
Yang JJ, El-Nahhal IM, Chung IS, Maciel GE (1997) J Non-Cryst Solid 202:19–39
El-Nahhal IM, El-Ashgar NM (2006) J Dispers Sci Technol 27(7):915–919
Davornic PR, Lenz RW (1990) High temperature siloxane elastomers. Huthing and Wepf Verlag, Basel, Heidelberg, New York
Vansant EF, Van Der Voort P, Vrancken KC (1995) Characterization and chemical modification of the silica surface. In: Studies in surface science catalysis. Elsevier Science, Amsterdam
Klonkowski AM, Koehler K, Schlaepfer CW (1993) J Mater Chem 3:105–110
Kłonkowski AM, Koehler K, Widernik T, Grobelna B (1996) J Mater Chem 6(4):579–584
Klonkowski AM, Widernik T, Grobelna B (2001) J Sol–Gel Sci Technol 20:161–180
Jovanovic JD, Govedarica MN, Dvornic PR, Popovic IG (1998) Polym Degrad Stab 61:87–93
Sakpal T, Kumar A, Kamble S, Kumar R, Chemical Engineering and Process Development (2012) Division CSIR-National Chemical Laboratory, Pune 411 008, India
Acknowledgments
The authors would like to thank the French Government for the Al-Maqdisi grant jointly with the Palestinian Ministry of Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, M.A., Abu Shaweesh, A.A., El-Ashgar, N.M. et al. Synthesis and characterization of immobilized-polysiloxane monoamine-thiol triacetic acid and its diamine and triamine derivatives. J Sol-Gel Sci Technol 78, 660–672 (2016). https://doi.org/10.1007/s10971-016-3980-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-3980-9