Adstract
Using one-pot synthesis, polysiloxane nanoparticles were successfully prepared from 3-mercaptopropyl trimethoxysilane. Next, the nanoparticles were photo-coated with furfuryl methacrylate by photo-initiated Thiol-Ene reaction for grafting furan substitutes on the surface of inorganic cores. In this process, polysiloxane core/poly(furfuryl methacrylate) shell nanoparticles were prepared at different irradiation times, and then their characteristic, morphological and thermal properties were investigated. Fourier transform infrared and solid-state 13C and 29Si nuclear magnetic resonance spectroscopies confirmed the successful photo-coating of the surfaces of nanoparticles. The size of coated nanoparticles prepared by irradiation for 24 h was measured as 186 nm in ethanol by dynamic light scattering, while the average diameter of 100 randomly selected pristine nanoparticles were determined at 42.8 nm by transmission electron microscopy. The coated polysiloxane nanoparticles were investigated by X-ray photoelectron spectroscopy and scanning electron microscopy, which revealed that they have the spherical core-shell structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In recent years, scientists have developed new technologies for the synthesis of new materials in nanoscale such as nanoparticles [1, 2]. In these initiatives, much attention has recently been focused on the development of hybrid particles, which are very promising materials made from an inorganic core and an organic molecule or macromolecule shell. The silica nanoparticles with hybrid structure are very popular because they can be easily synthesized and have modifiable organic surfaces in versatile types. The design and fabrication of hybrid nanoparticles is of a great interest in adhesives, coatings, and optoelectronic and biomedical diagnostic applications due to their tunable and unique properties that combine the best properties in both materials [3–7].
In recent years, the surface modification of silica nanoparticles is a prominent topic in material science. For scientists, it is important and considerable that a selected functional group is grafted onto the silica surface, covalently bonding with polymer matrix to improve affinity between the two phases and reducing the agglomeration of the filler [8, 9]. In this process, the silane agents are often considered as potential candidates for modifying the surface of nanoparticles directly [10, 11]. Among them, silane compounds with a thiol functional group such as 3-mercaptopropyl trimethoxysilane deserve a special attention due to the ability of leading the further functionalization via thiol-ene reaction [12–15]. Photoinitiated thiol-ene click reaction has gained an increasing attention in the architecture of advance polymers, since it benefits the rapid polymerization reactions with the high reaction rate and conversion yield even in the presence of oxygen [16–18]. Photoinitiated thiol-ene reaction with these highly desirable characteristics has been studied in a wide range of fields such as biomaterials, coatings, lithography, and electro-optics for decades [19–22]. However, the number of studies investigating to the thiol-ene reactions in nanoparticle systems has been still limited. In conjuction with the advantages of photoinitiated thiol-ene reactions, incorporating inorganic nanoparticles into photopolimerizable monomers with unsaturated bonds is a desirable method for generating high performance materials. In addition, the photocoating of the inorganic particle surface may reinforces nanomaterials and improves surface properties.
In this paper, new hybrid nanoparticles, containing a polysiloxane core synthesized by a self-condensation reaction with 3-mercaptopropyl trimethoxysilane (MPTMS) and a shell in which furfuryl methacrylate (FMA) including a furan group was polymerized by the thiol-ene reaction, which was initiated by the surface-localized radicals occurred on polysiloxane cores under UV irradiation, were prepared. Furan which is a molecule obtained from renewable resources as an abundant is able used for the preparation of innovative and eco-friendly high-performance polymers due to its controlled and improved thermal and thermomechanical properties. In literature, several studies report the utilization of furan-containing materials to meet the needs of chemistry science [23, 24]. Especially, the furan-based molecules have the ability to undergo the Diels-Alder reaction let them to applicate in a large field of studies referred to the intelligent materials such as shape memory and self-healing materials [25]. On the otherside, organic/inorganic nanoparticle structures including furan blocks are an interesting issue for the improvement of thermal performance of polymer networks. In this study, it was aimed to combine the properties of furan groups, with silica nanoparticle materials by applying the advantages of photoinitiated thiol-ene reaction in polysiloxane nanoparticle systems, with a new approach. it is predicted that this study is promising for going one step further in examining the applications of furan-based nanoparticles in future studies. On the other side, silica nanoparticles have been traditionally prepared from tetraethoxysilane (TEOS) by Stöber’s method. In the process of preparing the polymer-silica nanoparticle hybrid system with TEOS, it is required to an additional modification step for the surface functionalization that make it link to organic structure. For this reason, polysiloxane nanoparticles (SiNPs-SH) were prepared by the self-condensation reaction of the single source 3-mercaptopropyl trimethoxysilane in this study. Then, SiNPs-SH nanoparticles with mercaptopropyl chains that allow subsequent internal functionalization through thiol groups were photocoated by the photo-initiated thiol-ene polymerization of FMA, which was carried out at different irradiation times. In addition, the physical, thermal and morphological properties of polysiloxane nanoparticles were investigated.
EXPERIMENTAL
Materials
3-Mercaptopropyl trimethoxysilane (Sigma Aldrich), sodium hydroxide, hydrogen peroxide were used as received. Furfuryl methacrylate (Sigma Aldrich) was used after removal of the stabilizer by basic alumina. All solvents were analytical grade and supplied by Merck.
Preparation of Silica Nanoparticles
Thiolated silica particles were prepared using an one step method through the self-hydrolysis of organosilane in aqueous solution according to literature [13, 14]. MPTMS (0.75 mL, 0.07882 g) was mixed with 20 mL of DMSO, 0.5 mol/ L NaOH and 30 µL of 30% hydrogen peroxide (as an oxidizing agent) and stirred continuously using a magnete stirrer until transparent color is observed at room temperature (600 rpm) for 10 min. The reaction progressed for 24 h with stirring at the same speed at 40°C. After completion of the reaction, the solution was diluted by large amounts of ethanol and allowed to cool down to room temperature. The resulting precipitate was collected by centrifugation (3600 rpm, 20 min, RT) and washed three times upon ultrasonication (5 min, 40°C) using ethanol to remove non-reacted materials. The obtained particles (SiNPs-SH) were isolated in ethanol as suspension (15 mg/mL).
Surface-Initiated Photopolymerization
SiNPs-SH nanoparticles were photo-coated by the process according to the literature [14]. Approximately 30 mg of nanoparticles in 2 mL ethanol suspensions were added to 5 vol % of furfuryl methacrylate (1 mL in 20 mL of cyclohexane solution) under Ar atmosphere at room temperature. Photopolymerization reaction was carried out using a photoreactor consisting of 18 lamps at λ = 360 nm. Polymer grafted particles (SiNPs-PFMA) were sequentially centrifuged (3600 rpm) for 30 min and washed with ethanol and THF upon ultrasonication to remove non-reacted monomer.
Characterization
All the surface coated silica samples were agitated in THF for 30 min and then washed with ethanol and THF three times as described above. This procedure was carried out to remove any unreacted monomer from the samples. All the samples were dried over night at 90°C and stored in a desicator prior to analysis.
Dynamic light scattering (DLS) analysis was performed to reveal the average size of nanoparticles by three subsequent measurements calculated using the Stokes-Einstein relationship. These measurements were conducted with dilute dispersions of nanoparticles at 25°C in a Nano-S Zetasizer (Malvern Instruments). Nanoparticle concentration in dispersions was around 0.1 mg/mL in ethanol for each sample.
Transmittance infrared spectra were collected using a Perkin-Elmer 100 Fourier transform infrared (FTIR) Spectrometer. The spectra were obtained over the frequency range of 400–4000 cm–1 at a resolution of 4 cm–1 with an attenuated total reflectance (ATR) cell equipped a ZnSe single crystal.
Solid state cross polarization/magic angle spinning nuclear magnetic resonance (CP-MAS NMR) spectra were recorded on a JEOL ECZ 500 R (500 MHz) NMR Spectrometer. 13C and 29Si CP-MAS NMR spectra were recorded at 500 MHz using a 4 mm zirconia rotor (sample size ~ 50 mg). 13C and 29Si NMR spectra were externally referenced to Si(CH3)4 at room temperature (25°C).
TGA experiments were carried out on the samples placed in an alumina sample pan using a SEIKO TG/DTA 6300 analyzer. Samples ranging from 4 to 5 mg loaded into the alumina pan and sealed in the sample chamber. The samples were heated from 35 to 900°C under nitrogen atmosphere at the rate of 10°C/min.
The morphology of the surfaces of the nanomaterials was investigated with scanning electron microscopy (SEM) and scanning transmission electron microscopy (STEM) analyses using Inspect S50 and Philips-FEI XL30, respectively. To confirm the core-shell structure, it was studied with JEOL JEM-1400 PLUS Transmission Electron Microscopy (TEM).
The surface composition of the nanoparticles at different stages was obtained using a Thermo Scientific K-Alpha X-ray photoelectron spectrometer (XPS) with AlKα X-ray radiation with an energy of 150.0 eV and take off angle 90°.
RESULTS AND DISCUSSION
Synthesis and Characterization of Polyorganosiloxane Nanoparticles
It is known that high coverage of thiol groups on the surface of nanoparticles can be possible with MPTMS [13, 14]. The hydrolysis reaction of MPTMS is a sol–gel self-condensation, which is facile and efficient method [14]. This process related to the precursor chemistry of MPTMS offers several major advantages such as high monodispersity and well-defined controlled size [10, 12]. In contrast to the Stöber method [8], it does not need any other compounds such as tetramethyl orthosilicate (TMOS) or TEOS. On the other side, Khutoryanskiy et al. investigated various synthesis conditions to prepare micro- and nano-particles under room atmosphere and/or atmospheric oxygen gas, using different bases in a protic or aprotic solvent such as DMSO or water–ethanol mixture [13]. An aprotic medium made it possible to avoid solvent effects on the process of self-condensation of silane groups and to proceed with the reaction in a more controlled manner. It is also known that the presence of H2O2 increases the content of higher thiol groups and greatly affects the polydispersity of the particles [13]. For these reasons, in this study nanoparticles (SiNPs-SH) were synthesized by the self-condensation reaction of MPTMS using NaOH in the presence of hydrogen peroxide as an oxidizing agent in DMSO (Scheme 1). Then, these polysiloxane nanoparticles were photo-coated by the grafting-from method [14], which involves carrying out the polymerization of FMA via the photo-initiated thiol-ene reaction, performed by the initiation of polymerization reaction with surface-localized thiol radicals (Scheme 2). FMA is an important monomer enabling to incorporate furan moieties, which allow to have improved thermal properties and undergo characteristic reaction such as the Dield-Alder reaction, in a resulting material that has an organic and/or hybrid nature [1, 4, 25, 26].

Scheme 1.

Scheme 2.
The size of polysiloxane nanoparticles dispersed in ethanol were characterized by STEM, DLS and TEM as shown in Fig. 1. It was observed that SiNPs-SH nanoparticles have spherical shape. The diameters of 100 randomly selected nanoparticles were measured, and the average diameter was calculated as 42.8 nm. The mean diameter of SiNPs-SH nanoparticles measured by DLS was found to be 347.7 nm, which is higher than STEM. This difference was related to the hydrodynamic radius of the nanoparticles in the solvent medium. On the other side, the morphology of SiNPs-SH nanoparticles was studied by SEM (as shown in Fig. 2). The spherical nanoparticles showed the formation of densely packed aggregates as a result of the drying process.
Silica core-polymer shell particles, which were named SiNPs-PFMA, were prepared by the photoinitiated thiol-ene polymerization of FMA. Photopolymerization reaction was initiated by surface-bound thiyl radicals, which were directly formed through homolytic cleavage of –SH groups in the absence of any photoinitiator. In this initiation mechanism, radical species were only present at the particle surface, even if the particles were dispersed in the solvent. Then, this reaction was proceeded by attaching of these surface-localized radicals to FMA in excess of unsaturated species (as shown in Scheme 2). The preparation of SiNPs-PFMA nanoparticles were carried out at different reaction times to achieve the proper size particles with suitable polymer thickness in their shell.
TGA was studied to evaluate the amount of polymer at the particles’ surface. The mass loss of the neat and functionalized silica nanoparticles under nitrogen atmosphere as a function of temperature is shown in Fig. 3. While the pristine nano-silica total mass loss was determined as 45%, higher mass losses were observed in photo-coated nanoparticles due to the content of polymer. In the latter case, a first small decomposition step (3–5%) below 200°C amounting corresponded to the evaporation of absorbed water and/or organic solvent. The polymer degradation started at ~250°C. The nanoparticles lost approximately 62, 68, 71, 80 and 82% of their original mass after 6, 9, 24, 72, and 144 h of polymerization, respectively. Consequently, the organic content increased relative to the photoinitiated thiol-ene reaction time.
The size of SiNPs-PFMA nanoparticles photocoated at different irradiation times was measured by DLS in ethanol. An increase in hydrodynamic diameters from 137 to 1036 nm was detected with increase of duration of reaction (Table 1). The decrease of the size of the coated particles compared to pristine particles confirms that no significant agglomeration occurs after the surface modification. However, after 144 h the size of the particles and their dispersity (PDI) grew.
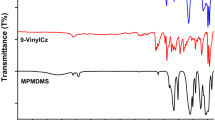
The success of photo-coating of SiNPs-SH nanoparticles was revealed by FTIR spectroscopy. Due to the significant changes in the chemical structure of the nanoparticles, the photocoating reaction can be easily followed by this method. The characteristic FTIR spectra of nanoparticles are shown in Fig. 4. A sharp and strong Si–O–Si stretching band (at ~1110 cm–1) was observed for all samples, indicating to unchanging core structure that shows transmittance in the ‘fingerprint’ region [14, 27]. Also, a band corresponding to silanol groups was observed at ~1026 cm–1 due to asymmetric Si–OH vibrations that occurred by the self-condensation reaction of MPTMS [8, 10]. On the other hand, characteristic bands appeared at ~745 and 1725 cm–1, referring to the furan and carbonyl groups assigned to the C=C and C=O stretching vibrations, respectively.
The morphology of photo-coated nanoparticles was investigated by STEM studies. STEM image clearly showed that the SiNPs-PFMA nanoparticles were nearly spherical shapes at nanometer size, although they were aggregated to a little degree (Fig. 5a). Then, photo-coated nanoparticles were analyzed by SEM after drying. As is seen their surface is densely covered with polymer (Fig. 5b).
Solid-state NMR analysis gives further structural information on the entities grafted onto the nanoparticle surface. Figure 6 presents 29Si NMR spectra of neat and photo-coated nanoparticles. Three signals were observed at 63, 55, and 47 ppm, corresponding to T4 + T4' (R'Si(OSi)3, crosslinked), T3 + T3' (R'Si(OSi)2OH, crosslinked) and T2 (R'Si(Si(SiO)2OH) species, respectively in which R' represents mercaptopropyl group in the MPTMS molecule. Crosslinked species (T4 + T4' and T3 + T3') are common in the surface of silica nanoparticles involving trifunctional alkoxysilanes [5, 28]. On the other hand, the 29Si NMR spectrum of SiNPs-PFMA nanoparticles showed remarkable differences. T2 and T3 + T3' signals dissappeared and the area of T4 + T4' signal decreased compared to the spectrum of SiNPs-SH nanoparticles due to the coated surface. In addition, 13C NMR spectra of neat and photo-coated nanoparticles were recorded (Fig. 7). Four peaks were observed in the spectrum of SiNPs-SH nanoparticles at 15 ppm (–CH2–SH), 31 ppm (–CH2–), 45 ppm (Si–CH2–) and 57 ppm (free –OCH3). In comparison, 13C NMR spectrum of SiNPs-PFMA nanoparticles clearly showed new signals according to the spectrum of neat ones. While the signals at 152, 131, and 114 ppm were assigned to the resonance on the furan ring, the peak at 180 ppm was due to the carbonyl peak of poly(FMA). Also, there were three prominent signals at 18, 36, and 61 ppm attributed to aliphatic groups from nanoparticle and photo-coated structures. Remarkably, the signal at 61 ppm is generally associated with C–S and C–O resonance [5]. The mentioned results from solid-state NMR techniques lead to the conclusion that the surface of silica nanoparticles was successfully photo-coated with furan entities on their surface.
XPS was used to investigate the photo-coated surface of nanoparticles. Figure 8 showed XPS spectra of pristine and photo-coated nanoparticles. The survey scan of SiNPs-SH (Fig. 8a) showed peaks corresponding to Si 2p (102 eV), Si 2s (153 eV), S 2p (164 eV), S 2s (230 eV), C 1s (228 eV) and O 1s (532 eV). The spectra of the coated nanoparticles (Figs. 8b–8e) indicated the change in the chemical composition due to the absence of thiol groups on the particle surface. The disappearance of the peaks of thiol groups was seen in all spectra without being affected by reaction times. Thus, the photoinitiated thiol-ene reactions were efficiently performed even at a minimum reaction time of 6 h. In addition, there were no Si 2s and/or Si 2p signals in the spectra after 72 and 144 h of the reaction. This result indicated that the coating thickness on the surface of polysiloxane nanoparticles increased during longer irradiation time.
CONCLUSIONS
Photo-coated nanoparticles were successfully prepared by photo-initiated thiol-ene polymerization of FMA under different irradiation times, after the synthesis of polysiloxane nanoparticles by the self-condensation process of MPTMS. FTIR spectra of polysiloxane nanoparticles demonstrated the changes in the characteristic bands of both inorganic and organic groups. TGA study revealed the presence of organic content bonded to polysiloxane nanoparticles according to the percent mass loss differences between SiNPs-SH and SiNPs-PFMA nanoparticles. The amount of photo-coated poly(FMA) slightly changed after 72 h of reaction time. 13C solid-state NMR studies revealed the presence of photo-coated poly(FMA) on the nanoparticle surface due to the signals assigned to the resonance of furan and carbonyl groups. Also, 29Si NMR results showed that in addition to decreased T4 + T4' signals, T2 and T3 + T3' signals of SiNPs-PFMA were disappeared. Moreover, X-ray photoelectron spectra proved that the reaction was successfully performed. Importantly, that pristine and photo-coated nanoparticles (up to 72 h) were in nanometer size and had spherical shape despite the presence of a little agglomeration.
REFERENCES
P. M. Ajayan, L. S. Schadler, and P. V. Braun, Nanocomposite Science and Technology (Wiley, New York, USA, 2003).
X. Liu, H. Liu, W. Zhou, H. Zheng, X. Yin, Y. Li, Y. Guo, M. Zhu, C. Ouyang, D. Zhu, and A. Xia, Langmuir 26, 3179 (2010).
G. Bissadi and R. Weberskirch, Polym. Chem. 7, 1271 (2016).
T. Engel and G. Kickelbick, Polym. Int. 63, 915 (2014).
P. Vejayakumaran, I. A. Rahman, C. S. Sipaut, J. Ismail, and C. K. Chee, J. Colloid Interface Sci. 328, 81 (2008).
G. S. Batıbay, E. O. Kazancioglu, E. Kose,and N. Arsu, Prog. Org. Coat. 152, 106113 (2021).
K. Khezri, H. RoghaniMamaqani, M. Sarsabili, M. Sobani, and S. A. Mirshafiei Langari, Polym. Sci., Ser. B 56, 909 (2014).
W. Stöber, A. Fink, and E. J. Bohn, J. Colloid Interface Sci. 26, 62 (1968).
S. S. Balamurugan, E. Soto-Cantu, R. Cueto, and P. S. Russo, Macromolecules 43, 62 (2010).
G. Kickelbick, D. Holzinger, S. Ivanovici, “Organically Functionalized Silica Nanoparticles,” in Materials Syntheses, Ed. By U. Schubert, N. Hüsing, and R. Laine, (Springerlink, Germany, 2008), pp. 127–133.
N. Karaca, Turk. J. Chem. 45, 761 (2021).
M. Nakamura and K. Ishimura, Langmuir 24, 5099 (2008).
G. S. Irmukhametova, G. A. Mun, and V. V. Khuntoryanskiy, Langmuir 27, 9551 (2011).
C. Kurttner, P. C. Maier, C. Kunert, H. Schlaad, and A. Fery, Langmuir 29, 16119 (2013).
H. Zhou and H. Schlaad, Polym. Chem. 53, 1260 (2015).
C. E. Hoyle, A. B. Lowe, and C. N. Bowman, Chem. Soc. Rev. 39, 1355 (2010).
C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed. 49, 1540 (2010).
W. Xi, T. F. Scott, C. J. Kloxin, and C. N. Bowman, Adv. Funct. Mater. 24, 2572 (2014).
A. B. Lowe, Polym. Chem. 1, 17 (2010).
M. A. Tasdelen and Y. Yagci, Angew. Chem., Int. Ed. 52, 5930 (2013).
G. Temel, N. Karaca, and N. Arsu, J. Polym. Sci., Part A: Polym. Chem. 48, 5306 (2010).
Z. Dogruyol, G. Temel, S. K. Dogruyol, O. Pekcan, and N. Arsu, Prog. Org. Coat. 76, 944 (2013).
N. Guigo, A. Mija, R. Zavaglia, L. Vincent, and N. Sbirrazzuoli, Polym. Degrad. Stab. 94, 908 (2009).
A. Gandini and M. N. Belgacem, Prog. Polym. Sci. 22, 1203 (1997).
A. Gandini, Prog. Polym. Sci. 38, 1 (2013).
B. Oktay and E. Çakmakçı, Polymer 131, 132 (2017).
V. Kumar, N. Misra, J. Paul, Y. K. Bhardwaj, N. K. Goel, S. Francis, K. S. S. Sarma, and L. Varshney, Prog. Org. Coat. 76, 1119 (2013).
K. Albert, B. Pfleıderer, E. Bayer, and R. Schnabel, J. Colloid Interface Sci. 142, 142 (1991).
Funding
Yalova University and Yalova University Scientific Research Projects Coordination Unit are acknowledged for the financial support (Project no. 2015/BAP/120).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nurcan Karaca Preparation of Photocoated Polysiloxane Nanoparticles from 3-Mercaptopropyl Trimethoxysilane with Furan Substitues by the Photoinitiated Thiol-Ene Reaction. Polym. Sci. Ser. B 64, 606–615 (2022). https://doi.org/10.1134/S1560090422700397
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090422700397