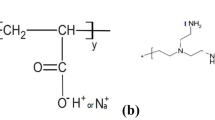
Abstract
Compound crosslinked hydrolyzed polyacrylic amide (HPAm) weak gels were synthesized by an in situ crosslinking method using Cr(III) acetate and methanal as crosslinkers. The weak gels were crosslinked by both covalent bonds and coordination bonds. A Haake MARS III rotational rheometer was used to measure viscosities to monitor gelation process and evaluate the sensitive of six main factors including polymer concentration etc. An orthogonal experiment (OA25 (56) matrix) was conducted to help to evaluate the importance of the factors, and determine the optimal level and combination of factors. ESEM was employed to image the morphologies of gels to assist to analyze crosslinking mechanism. We found that the six factors have significant influences on gelation properties. The importance of factors for viscosity of 40 days followed by polymer concentration, weight ratio of polymer to crosslinker, NaCl concentration, thiourea concentration, resorcin concentration and methanal concentration. The reasons why these factors had such effect were analyzed. On the basis of the analysis of gelation process and ESEM morphology, we preliminarily concluded that crosslinking mainly happened among different polymer molecules and crosslinking by coordination bonds prevails in early stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Most production is achieved by water or gas injection to sweep reservoir and displace oil towards production wells in oil and gas production process. However, the lower viscosity water or gas inevitably towards the higher permeability pathways and large quantities of crude oil remain by-passed, thus a low oil recovery (typically only 30–40 %) is achieved, which is one of the most pressing issues facing operators worldwide [1]. Conformance control processes, mainly including near-wellbore water shut off on producer wells and profile modification treatments on injector wells, have already been attempted to solve this problem to improve oil recovery. Polymer gels are attracting increasing interest for conformance control implemented to improve petroleum production [2].
However, the performances of many current polymer gels are sensitive to oilfield environments and interferences, especially pH and salinity and temperature [3–5]. Gel containing HPAm and Cr(III) acetate has been one of the most widely applied gels because HPAm–Cr(III) acetate gel has good stability at over a wide range of temperature and pH (from 3.3 to 12.5) [6, 7]. However, the gelation rate of the HPAm gels only crosslinked by the simple Cr(III) in solution is too fast to control. It is necessary to effectively delay gelation rates [8]. The introduction of acetate complexes of Cr(III) makes the rate of the crosslinking reaction greatly reduce [6, 9–11]. The Cr(III)/HPAm crosslinking reaction displays a typical coordination chemical reactivity. A Cr(III) based delayed-gelation technology has been developed based on the idea that the polymer crosslinking reaction is fundamentally a ligand-exchange process [12, 13]. And, acetate is a thermodynamically weaker ligand for Cr(III) than HPAm, the gelation time for Cr(III) acetate is greater than that obtained with the aquated Cr(III) [2]. Tackett et al. [14] confirmed the existence of the cyclic chromium trimer in chromium acetate solution. This cyclic chromium trimer has been considered responsible for the interaction between Cr(III) and polymer carboxylate groups [15, 16]. Therefore, in our study, Cr(III) acetate is chosen to be crosslinker. And, the cyclic chromium trimer is guaranteed to be the dominant species in Cr(III) acetate solution by using a proper method [14].
Nevertheless, there are still some issues when these HPAm/Cr(III) acetate polymer gels are applied for in-depth profile control and oil displacement in low temperature and high salinity reservoirs, such as bad long-term stability (only crosslinked by metallic-ion) and bad salt tolerance. These two properties are urgently needed to be addressed.
1.1 Improve long-term stability
The Cr(III)/HPAm crosslinking reaction displays a typical coordination chemical reactivity [2]. The long-term stability of the gels crosslinked by coordination bonds is not good compared with that of the gels crosslinked by covalent bonds. To improve the stability, methanal as another crosslinker was added into the HPAm/Cr(III) acetate gel solutions to form compound crosslinked weak gels (crosslinked by coordination bonds and covalent bonds).
1.2 Increase salt tolerance
It has been confirmed that the presence of salt can lead to the syneresis of gels [13, 17]. The syneresis of gels will badly shorten the validity of gels. Therefore, it is essential to improve salt tolerance to extend the application range of HPAm/Cr(III) acetate gel to high salinity reservoirs. In this study, amphoteric HPAm is used for forming weak gel to ease syneresis.
This paper aims at present a low-temperature compound crosslinked weak gel which gives appropriate gelation rates, gel stability and salt-resistance at the same time. In the present case, this study focuses on the following contents: (1) investigating the effects of the six main factors on gel performance at 32 °C, including polymer concentration, weight ratio of polymer to crosslinker, aging time of Cr(III) acetate solutions, NaCl concentration, additives concentration (methanal, resorcinol and thiourea); (2) determining the importance of all the parameters on gelation performance by range analysis and mean value, and giving the optimal combination of factors; (3) analyzing the ESEM morphology of gels and crosslinking mechanisms.
2 Experimental section
2.1 Chemicals and reagents
HPAm (Mw = 2.5 × 107, 25 % hydrolyzed, 90 % solid content) was purchased from Beijing Hengju, China. Chromic chloride hexahydrate (CrCl3·6H2O, 99 %), sodium acetate (NaAC, 99.0 %), methanal (HCHO, 37–40 %), resorcinol (C6H6O2, 99.5 %), thiourea (CH4N2S, 99.0 %), sodium chloride (NaCl, 99.5 %) were purchased from Kelong, Chengdu, China.
2.2 Synthesis of Cr(III) acetate solutions
Cr(III) acetate solutions were prepared with chromic chloride hexahydrate and sodium acetate at the acetate-to-chromium ratio of 3:1 [14] and were placed in a constant temperature water bath at 60 °C for 1 h and aged for different times (3 h–10 days) at room temperature.
2.3 Preparation of polymer solution
Polymer stock solutions were prepared in distilled water or salt water with different NaCl concentration ranging from 5,000 to 100,000 mg/L using a method described in the literature [18]. The polymer powder was slowly and evenly added into container containing distilled or salt water with magnetic stirring. Stop stirring when the polymer powder was sufficiently dissolved. The polymer stock solutions were diluted to the required concentration.
2.4 Synthesis and evaluation of weak gel
Gels were prepared in jars 8 cm in height and 80 mL in volume. To guarantee a uniform mixture of all elements, the water used (distilled water or salt water), crosslinker, additives (methanal, resorcinol, thiourea) and polymer solution were added in turn according to fixed proportion (changed according to different evaluation index) to give 30 mL of final gel solution. The samples were aged at 32 °C in an oven thermostat. A Haake MARS III rotational rheometer (Germany) was used to measure viscosity at the applied shear rate of 7.34 s−1 at 32 °C. Gelation rates can be observed through the viscosity versus time curve. The time when the viscosity of gel solutions was measured at first was recorded as zero time in the viscosity versus time curve. ESEM (Quanta 450 FEG, FEI, USA) was used to image the morphology of gels.
2.5 Orthogonal experimental design
Orthogonal experimental design has been proved to be an effective chemometric method for optimization [19]. In this study, considering the validity of gels, the gels viscosity for 40 days was taken as evaluating index. Six factors affecting gelation properties were investigated: polymer concentration (factor A), ratio of polymer to crosslinker (factor B), salinity (factor C), thiourea concentration (factor D), methanal concentration (factor E), resorcin concentration (factor F). An OA25 (56) matrix, which is an orthogonal array of six factors and five levels, was employed to assign the considered factors and levels as shown in Table 1.
There are two important parameters in range analysis [20]: Kji and Rj. Kji is defined as the sum of the evaluation indexes of all levels (i, i = 1, 2, 3, 4, 5) in each factor (j, j = A, B, C, D, E, F) and kji (mean value of Kji) is used to determine the optimal level and the optimal combination of factors. The optimal level for each factor could be obtained when kji is the nearest to expected value. Rj is defined as the range between the maximum and minimum value of kji and is used for evaluating the importance of the factors, i.e. a larger Rj means a greater importance of the factor. In our OA25 (56) matrix, take factor B as a calculation example to obtain the kBi and RB:
where KBi is the K value of the i level of the factor of B; and Vi (i = from 1 to 25) is the value of the result of the trail No. 1. when viscosity for 40 days is as evaluating index. The other Kji and Rj values of the factors can be calculated by the same steps.
3 Results and discussion
3.1 Effect of polymer concentration
The polymer concentration is critical for gels properties. Previous studies on the effect of polymer concentration more focused on changing polymer concentration and keeping a constant crosslinker concentration [5, 21]. However, in our single-factor experiments, a constant weight ratio of polymer to crosslinker was kept at 8:1 to more accurately obtain the influence of polymer concentration. Figure 1 shows the effect of polymer concentration on gelation rates. The results indicated that the gelation rates significantly increased with increasing polymer concentration when polymer concentration was over 1,500 mg/L, while HPAm of 1,000 mg/L failed to gel for about 40 h, which demonstrates this gel system needs a minimum polymer concentration that is called critical overlap concentration (COC). The COC value is between 1,000 and 1,500 mg/L for HPAm. The weak gels were formed over a broad range of polymer concentrations above COC. The effect of polymer concentration may be explained through the chemistry of polymer crosslinking reaction that is fundamentally a ligand-exchange process [12]. This chemistry is written in the following equation:

Effect of polymer concentration on gelation rates. Experiment conditions: polymer (HPAm) concentration range was from 1,000 to 4,000 mg/L, polymer/crosslinker weight ratio 8:1, Cr(III) acetate solutions (acetate-to-chromium molar ratio of 3:1) first aged for 1 h at 60 °C, then aged for 3 days at room temperature, NaCl concentration 10,000 mg/L, no additives (methanal, resorcinol and thiourea), T = 32 °C
The chemistry of polymer crosslinking reaction was proposed based on the ligand-exchange process as Cr(III) acetate complex is dominated in Cr(III) acetate solutions. Cr(acetate)3 indicates Cr(acetate)3 complex, P-CO2 − indicates polymer-bound carboxylate group. The concentration of P-CO2 − increases with increasing polymer concentration. Thus, the increased P-CO2 − promotes the reaction to generate the crosslink complex, which further quickens overall reaction rates. However, the increasing velocity of reaction rates decreases when the polymer concentration increases to a certain value (about 3,500 mg/L). It can be reasonably presumed that the overall reaction will finally reach the equilibrium. Furthermore, the effect of polymer concentration also can be explained from the perspective of crosslink point. There are strong attractive forces between polymer macromolecules. As the polymer concentration increases, the molecules per unit volume increases and the distance apart of the molecules becomes smaller, which strengthens intermolecular attractive forces, and then, the collision probabilities increase during the heat motion of polymer molecules. Thereby, the intertwisting of different molecules, which gives rise to a network structure, is strengthened. Consequently, the effective crosslink points increase, which promotes crosslinking reactions. The concept of increasing crosslink points is consistent with the idea that we explained the effect of polymer concentration through the proposed chemistry.
3.2 Effect of weight ratio of polymer to crosslinker
As shown in Fig. 2, weight ratio of polymer to crosslinker has a significant effect on gelation rates. The gelation rates decreased with increasing weight ratio of polymer to crosslinker. There was even no detectable gel formed as the weight ratio increased up to 20:1, which still can be explained by the chemistry (presented in the Sect. 3.1). The ionic acetate, Cr+3 and Cr+3-complex exist simultaneously in chromium acetate solutions, notwithstanding Cr(acetate)3 complex is the dominated species [14]. The active Cr+3 will first complex with polymer-bound carboxylate group. This complex reaction will promote the Cr(acetate)3 complex to release Cr+3 to complex with carboxylate group. Finally, theoretically, all Cr+3 existed in any form will participate in the complex reaction as enough carboxylate groups are available in gel solutions. But the weight ratio of 20:1 failed to gel. This implies that there is a lowest concentration limit of total Cr+3 in Cr(III) acetate solutions to build a complex network.
Effect of weight ratio of polymer to crosslinker on gelation rates. Experiment conditions: polymer/crosslinker weight ratio varied from 5:1 to 20:1, Cr(III) acetate solutions (acetate-to-chromium molar ratio of 3:1) first aged for 1 h at 60 °C, then aged for 3 days, HPAm concentration 2,000 mg/L, NaCl concentration 10,000 mg/L, no additives, T = 32 °C
3.3 Effect of the aging time of crosslinker
Crosslinkers were prepared at different conditions as follows: (1) crosslinker-1: acetate-to-chromium molar ratio of 4:1, aging 1 h at 60 °C, 3 h at room temperature; (2) crosslinker-2, crosslinker-3 and crosslinker-4: acetate-to-chromium molar ratio of 3:1, first all aging 1 h at 60 °C, then aging 10, 8 days, and 3 h, respectively. As shown in Fig. 3, the gelation rates decreased with increasing aging time at room temperature. The effect of aging time is relevant to the process of forming stable cyclic trimer in Cr(III) acetate solutions. It was found that a green cyclic chromium trimer that contains six acetates and a central trigonal oxygen is the dominant species when acetate and Cr(III) are in solutions in molar ratio of 3:1 and subjected to sufficient time or temperature to allow them to approach equilibrium [14]. We can clearly see that, from Fig. 3, gel solution with crosslinker-4 (aged for 3 h at room temperature) showed a quick gelation rate, which indicates a time of 3 h was too short to form the cyclic trimer, and most of acetate and Cr(III) remained free. However, gelation rates became slow when the aging time of crosslinker-2 and crosslinker-3 was prolonged to 8 days and 10 days, respectively. Gelation rates reduced with increasing aging time shows that the formation of cyclic trimer is a slow process and it requests enough time to make the cyclic trimer to be the dominate species in solution under our experimental conditions, which is consistent with the results obtained by Tackett et al. [14].
The effect of aging time of crosslinker on gelation rates. Crosslinker-1: acetate-to-chromium molar ratio of 4:1, aging 1 h at 60 °C, 3 h at room temperature; crosslinker-2, crosslinker-3 and crosslinker-4: acetate-to-chromium molar ratio of 3:1, first all aging 1 h at 60 °C, then aging 10 days, 8 days, and 3 h, respectively. Experiment conditions: polymer/crosslinker weight ratio 8:1, HPAm concentration 2,000 mg/L, NaCl concentration 10,000 mg/L, no additives, T = 32 °C
3.4 Effect of NaCl
The salt water with different NaCl concentration varying from 0 to 100,000 mg/L was used to determine the effect of NaCl concentration on gelation rates as shown in Fig. 4. Gel solutions failed to gel or formed a low viscosity gel with a low crosslinking degree when NaCl concentration was lower than 5,000 mg/L. The gelation rates increased with increasing NaCl concentration from 0 to 50,000 mg/L. However, gelation rates with NaCl concentration of 100,000 mg/L showed a decrease trend compared with that of NaCl concentration of 50,000 mg/L. These results are somewhat inconsistent with the previous studies. Shriwal et al. [3] and Broseta et al. [4] reported that gelation rates decreased with increasing salinity, Albonico et al. [13] and Romero-Zeron et al. [13, 22] demonstrated that gelation rates were markedly shorten in salinity water, and Sydansk [6] indicated that gelation rates increased somewhat with salinity between 0 and 1,000 mg/L NaCl, however, were rather independent of salinity for concentrations exceeding 1,000 mg/L. But we preferred to believe that different NaCl concentration ranges have different effects on gelation rates. According to our experiments, we considered that salt water with a certain concentration range NaCl concentration (0–50,000 mg/L) can increase gelation rates, and there is a critical value for NaCl concentration, over which the presence of salt will delay gelation. Such influence of of electrolytes (i.e., NaCl) on gelation rates can be explained by two main effects.
Effect of NaCl concentration on gelation rates. Experiment conditions: NaCl concentration varied from 0 to 100,000 mg/L, polymer/crosslinker weight ratio 8:1, HPAm concentration 2,000 mg/L, Cr(III) acetate solutions (acetate-to-chromium molar ratio of 3:1) first aged for 1 h at 60 °C, then aged for 3 days, no additives, T = 32 °C
3.4.1 Promoting ionization
The introduction of Na+ makes weak acid (–COOH) ionization balance toward to complete ionization of the strong electrolyte (–COONa), which increases the content of carboxylate ions (–COO−) in the polymer solution. The increased carboxylate ions will promote crosslinking.
3.4.2 Electrostatic shielding effect
Sodium ions (Na+) screen the electrostatic (repulsive) interactions between the negative (acrylate) charges carried by the polymer and lead to chain shrinking (Fig. 5). When screening effect of the electrostatic interactions is weak and chain shrinking is not severe, repulsive interactions among carboxylate ions still prevails. The shrinking on some level reduces the distance of the carboxyl group of different polymer molecule chains and higher association degree predominates between polymer molecules. This will promote crosslinking reaction. However, when screening effect of the electrostatic interactions is strong, molecules chains shrinking is very severe and most of carboxyl groups are buried inside molecules coils shrinked. Consequently, crosslinking is delayed.
The influence of NaCl concentration on gelation rates depends on both the two effects.
3.5 Effect of additives
This section mainly presents the effect of the three additives, methanal, resorcin and thiourea to gelation rate and gel strength. Methanal was used as an organic crosslinker. Resorcin was added to assist to adjust the gelation rates. Figures 6 and 7 show the effect of methanal and resorcin on gelation rates, respectively. We can see that, from Fig. 6, methanal partly increased gelation rates and gel viscosities after about 100 h. This is because a covalent cross-linking reaction occurred between methanal and acylamino in HPAm molecule chains. As shown in Fig. 7, resorcin had little influence on gelation rates before 100 h and gel viscosities. However, resorcin clearly decreased galtion rates and delayed gelation times when resorcin concentration is higher than 50 mg/L, which can be explained by the reaction between methanal and resorcin. The 1,3-hydroxyl groups on the benzene ring make the 2,4,6-carbon atom very active and easily react with methanal to generate hydroxymethylated resorcinol. The reaction, to some extent, delays the covalent cross-linking reaction occurred between methanal and acylamino and prolongs gelation times. Thiourea was used to reduce oxygen-induced decomposition of the HPAm. The effect of thiourea on gel viscosities is as shown in Fig. 8. In the beginning, the viscosity for 30 days (represent gel stability in some degree) gradually increased with increasing thiourea concentration. Then, the increasing extent reduced, and the viscosity began to decrease when thiourea concentration reached upto a certain value. It implied that there is a preferred concentration value existed between 60 and 100 mg/L.
Effect of methanal concentration on gelation rates. Experiment conditions: methanal concentration varied from 0 to 100 mg/L, polymer/crosslinker weight ratio 8:1, HPAm concentration 2,000 mg/L, Cr(III) acetate solutions (acetate-to-chromium molar ratio of 3:1) first aged for 1 h at 60 °C, then aged for 3 days, NaCl concentration 10,000 mg/L, T = 32 °C
Effect of resorcin concentration on gelation rates. Experiment conditions: resorcin concentration varied from 0 to 100 mg/L, methanal concentration 50 mg/L, polymer/crosslinker weight ratio 8:1, HPAm concentration 2,000 mg/L, Cr(III) acetate solutions (acetate-to-chromium molar ratio of 3:1) first aged for 1 h at 60 °C, then aged for 3 days, NaCl concentration 10,000 mg/L, T = 32 °C
Effect of thiourea concentration on gel viscosity. Experiment conditions: polymer/crosslinker weight ratio 8:1, HPAm concentration 2,000 mg/L, Cr(III) acetate solutions (acetate-to-chromium molar ratio of 3:1) first aged for 1 h at 60 °C, then aged for 3 days, NaCl concentration 10,000 mg/L, T = 32 °C
3.6 Range analysis
According to the OA25 (56) matrix, twenty-five experiments were conducted. The results of the viscosities for 40 days as evaluating index are shown in Table 2. We can observe, from Table 2, that the viscosities for 40 days varied from 14.4 to 98,200 mPa s. The kji and Rj for every factors at different levels were calculated according to the method as mentioned in Sect. 2.5. As the viscosity for 40 days is the evaluating index, the higher viscosity value is preferred considering field application requirements where a high viscosity means a good stability and validity when the gel solutions are injected into reservoirs for 40 days. Therefore, the optimal level and the optimal combination of factors (A3B1C4D5E5F3) can be determined base on the highest mean value of viscosity for 40 days for each level in Table 2 as follows: polymer concentration is 2,000 mg/L (42,032.06), weight ratio of polymer to crosslinker is 5:1 (41,202.88), salinity is 50,000 mg/L (42,962.88), thiourea concentration is 100 mg/L (38,319.80), methanal concentration is 100 mg/L (28,597.44), resorcin concentration is 80 mg/L (30,400.15). The sub-optimal combination is A4B2C3D2E2F4, as polymer concentration is 2,500 mg/L (35,840.00), weight ratio of polymer to crosslinker is 8:1 (38,442.88), salinity is 20,000 mg/L (24,542.88), thiourea concentration is 40 mg/L (30,837.30), methanal concentration is 40 mg/L (26,265.76), resorcin concentration is 100 mg/L (30,317.30). The Rj indicates the factors’ significance. A larger Rj means the factor has a bigger impact on evaluating index. The Rj for each factor is depicted in Fig. 9. The factor’s level of significance followed by: polymer concentration, weight ratio of polymer to crosslinker, salinity, thiourea concentration, resorcin concentration and methanal concentration.
3.7 ESEM morphology and crosslinking mechanism analysis
Panels (a) and (b) in Fig. 10 show the ESEM morphologies images of HPAm prepared in salt water. Panels (c), (d) and Panels (e), (f) in Fig. 10 show the ESEM morphologies images of gels crosslinked with only Cr(acetate)3 complex and Cr(acetate)3 complex + methanal + resorcinol, respectively. On the basis of the morphologies images of gels, one can see that crosslinking reaction mainly happened among different polymer molecules. This indirectly demonstrates that the HPAm molecules chains can well stretched in salt water with a NaCl concentration of 10,000 mg/L. However, the morphologies of gels [panels (e) and (f)] in which methanal and resorcin existed has a higher degree of tacticity than that of gels [panels (c) and (d)]. We found that, in evaluating process, these well-aligned structure gels exhibited a better stability and well less prone to syneresis compared with those gels without methanal and resorcin. We supposed that the improved stability can be partly attributed to covalent bonds generated in crosslinking complex. There are two reactions in the process of crosslinking reaction in gel solutions containing HPAm, synthesized Cr(III) acetate, methanal and resorcin. On the one hand, Cr(III) acetate bonded chemically with carboxylate functional groups of HPAm by coordination bonds. On the other hand, methanal or hydroxymethylated resorcinol (formed through reaction between methanal and resorcin) bonded chemically with amides groups of HPAm by covalent bonds. But, only the former reaction occurred in gel solutions without methanal and resorcin. When evaluating the effect of methanal and resorcin, we learned that both methanal and resorcin had little influence on gelation process before 100 h. Therefore, we assumed that Cr(III) acetate complexed with carboxylate functional groups dominated in the early stages, then, the two crosslinking reaction simultaneously dominated. The covalent crosslinking produces elastomeric properties with potentially high strengths, which make gels have a stronger ability to resist external forces. The improved viscoelasticity and stability of gels will provide a better effect for profile control in field application. The crosslinking happens by forming both covalent and coordination bonds that link one polymer chain to another when gel solutions are injected into reservoirs. As a result, the bigger molecular aggregate is formed. The polymers lose some of their ability to move freely as individual polymer chains and the whole fluid turns into gels by linking the chains together. When gel solutions are displaced by subsequent fluid, they will flow and migrate in porous media in the form of the micellaes with the biggest aggregating intensity, rather than migrate as single small polymer molecular that move forward along mainstream line like a ‘shuttle’. There must be stretching, creeping and recovery effect due to shear flow when these flexible micellaes through pore medium, which will exhibit a higher viscoelastic effect compared with the gels formed only by coordination bonds [panels (c) and (d)].
a, b are the ESEM morphologies images of HPAm salt-resistant comb-shape polymer prepared in salt water with a NaCl concentration of 10,000 mg/L. c, d are the ESEM morphologies images of HPAm/synthesized Cr(III) acetate gel prepared under the condition: salt water with a NaCl concentration of 10,000 mg/L, HPAm concentration 2,000 mg/L, weight ratio of polymer to crosslinker 8:1, thiourea concentration 50 mg/L, T = 32 °C. e, f are ESEM morphologies images of HPAm/synthesized Cr(III) acetate gel prepared under the condition: salt water with a NaCl concentration of 10,000 mg/L, HPAm concentration 2,000 mg/L, weight ratio of polymer to crosslinker 8:1, thiourea concentration 50 mg/L, methanal concentration 50 mg/L, resorcin concentration 50 mg/L, T = 32 °C
Obviously, the addition of methanal and resorcin has already made the mechanical and chemical properties of the polymer gels typically change compared with that of those gels without methanal and resorcin. However, for the present study, we have difficulty in determining why the morphologies of gels were changed significantly with adding of methanal and resorcin. The kinetics and mechanism of the two crosslinking reactions in gel solutions containing HPAm, synthesized Cr(III) acetate, methanal and resorcin still need to be further investigated by combining other means, such as, H NMR, UV spectrophotometry [23].
4 Conclusion
A novel HPAm–Cr(III) acetate-methanal weak gel was synthesized at low temperature for in-depth profile control and oil displacement. The combination of chelating crosslinker (Cr(III) acetate) and covalent crosslinker gives an appropriate gelation time and gel strength, which solves the problem of crosslinking by only covalent bonds giving a too low gelation rate at low temperature and improves the stability of gels compared with gels only crosslinked by coordination bonds.
Both single-factor experiments and orthogonal experiments demonstrated that these parameters evaluated have a significant influence on gel properties. The factors’ level of significance followed by: polymer concentration, weight ratio of polymer to crosslinker, NaCl concentration, thiourea concentration, resorcin concentration and methanal concentration. The gel system suited to a low-temperature (32 °C) reservoir was determined based on single-factor and orthogonal experiments as follows: HPAm concentration is 2,000–3,000 mg/L, weight ratio of polymer to crosslinker is 8:1–10:1, aging time of crosslinker ≥10 days, thiourea concentration is 60–100 mg/L, methanal concentration is 20–60 mg/L, resorcin concentration is 80–100 mg/L. These variables may be manipulated to achieve economic and gel performances optimality. The gel system can be adjusted according to the salinity of reservoirs. Gelaiton rates and gel strength can be controlled mainly by changing HPAm concentration and weight ratio of polymer to crosslinker. Gelation rates in later stage reaction can be fine-tuned by adjusting resorcin concentration.
According to the results obtained, we preliminarily confirmed that weak gels were crosslinked by two reactions: (1) Cr(III) acetate acetates bonded chemically with carboxylate functional groups of HPAm by coordination bonds; (2) methanal or hydroxymethylated resorcinol bonded chemically with amides groups of HPAm by covalent bonds. Cr(III) acetate complexed with carboxylate functional groups predominated in the early stages, then, the two crosslinking reaction were simultaneously occurred. The introduction of covalent bonds improves the stability of gels and makes a longer validity. The addition of methanal and resorcin makes the morphologies of gels been changed significantly and a well-aligned structure was obtained, which changes the chemical properties of the polymer gels. Furthermore, morphologies images of gels shows crosslinking reaction mainly happened among different polymer molecules. This weak gel can partly extend the application range of polymer weak gel to those reservoirs that have a lower temperature and higher salinity, which has a significant meaning to enhanced oil recovery for long time water/gas flooding reservoirs.
References
Maitland GC (2000) Oil and gas production. Curr Opin Colloid Interface Sci 5(5):301–311
Lockhart TP (1994) Chemical properties of chromium/polyacrylamide gels. SPE Adv Technol Ser 2(2):199–205
Shriwal, P, Lane R (2012) Impacts of timing of crosslinker addition on water shutoff polymer gel properties. In: SPE improved oil recovery symposium
Broseta D, Marquer O, Blin N, Zaitoun A (2000) Rheological screening of low-molecular-weight polyacrylamide/chromium (III) acetate water shutoff gels. In: SPE/DOE improved oil recovery symposium
Simjoo M, Vafaie Sefti M, Dadvand Koohi A, Hasheminasab R, Sajadian V (2007) Polyacrylamide gel polymer as water shut-off system: preparation and investigation of physical and chemical properties in one of the Iranian oil reservoirs conditions. Iran J Chem Chem Eng 26(4):99–108
Sydansk RD (1990) A newly developed chromium(III) gel technology. SPE Reserv Eng 5(3):346–352
Natarajan D, McCool CS, Green DW, Willhite GP (1998) Control of in situ gelation time for HPAAM-chromium acetate systems. In: SPE/DOE improved oil recovery symposium
Cheng M, Wang C, McCool CS, Green DW, Willhite GP (2005) Modeling of pre-gel aggregate growth during the gelation of a polyacrylamide-chromium (III) acetate gel system using the theory of branching processes. In: SPE international symposium on oilfield chemistry
Sydansk R D (1988) A new conformance-improvement-treatment chromium (III) gel technology. In: SPE enhanced oil recovery symposium
Sydansk RD, Argabright PA (1987) Conformance improvement in a subterranean hydrocarbon-bearing formation using a polymer gel. U.S. Patent No. 4,683,949
Sydansk RD (1993) Acrylamide-polymer/chromium (III)-carboxylate gels for near wellbore matrix treatments. SPE Adv Technol Ser 1(1):146–152
Lockhart TP, Albonico P (1994) New chemistry for the placement of chromium (III)/polymer gels in high-temperature reservoirs. SPE Prod Facil 9(4):273–279
Albonico P, Burrafato G, Alberto D, Lockhart TP (1993) Effective gelation-delaying additives for Cr+3/polymer gels. In: SPE international symposium on oilfield chemistry
Tackett JE (1989) Characterization of chromium(III) acetate in aqueous solution. Appl Spectrosc 43(3):490–499
Shu P (1989) Gelation mechanism of chromium(III). In: Oil field chemistry ACS symposium series Ed. Borckard JK, Yen TF, 137–144
Prud’homme R, Uhl J, Poinsatte J (1983) Rheological monitoring of the formation of polyacrylamide/Cr+3 gels. Old SPE J 23(5):804–808
Vargas-Vasquez SM, Romero-Zerón LB (2008) A review of the partly hydrolyzed polyacrylamide Cr(III) acetate polymer gels. Pet Sci Technol 26(4):481–498
Littmann W (1988) Polymer flooding, vol 24. Elsevier Science
Wu J, Lee HK (2005) Orthogonal array designs for the optimization of liquid–liquid–liquid microextraction of nonsteroidal anti-inflammatory drugs combined with high-performance liquid chromatography-ultraviolet detection. J Chromatogr A 1092(2):182–190
Wu X, Leung DY (2011) Optimization of biodiesel production from camelina oil using orthogonal experiment. Appl Energy 88(11):3615–3624
Jia H, Pu WF, Zhao JZ, Jin FY (2010) Research on the gelation performance of low toxic PEI cross-linking PHPAM gel systems as water shutoff agents in low temperature reservoirs. Ind Eng Chem Res 49(20):9618–9624
Romero-Zeron L, Hum F, Kantzas A (2008) Characterization of crosslinked gel kinetics and gel strength by use of NMR. SPE Reserv Eval Eng 11(3):439–453
Vargas-Vasquez SM, Romero-Zerón LB, Macgregor R, Gopalakrishnan S (2007) Monitoring the cross-linking of a HPAm/Cr(III) acetate polymer gel using 1H NMR, UV spectrophotometry, bottle testing, and rheology. Int J Polym Anal Charact 12(5):339–357
Acknowledgments
This research is financially supported by science and technology innovation talent project (2014-070) and scientific research project of education department (14ZB0042), Sichuan province. The authors express their sincere appreciation to the financial supports.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jin, FY., Yuan, CD., Pu, WF. et al. Investigation on gelation process and microstructure for partially hydrolyzed polyacrylic amide (HPAm)–Cr(III) acetate–methanal compound crosslinked weak gel. J Sol-Gel Sci Technol 73, 181–191 (2015). https://doi.org/10.1007/s10971-014-3509-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-014-3509-z