Abstract
The direct production of 99mTc through (p, 2n) reaction on natural molybdenum target and enriched 100Mo target was investigated. Separation of technetium radionuclide from the irradiated 100Mo target by a new method using Dowex-1 ion exchange resin as well as by the standard solvent extraction (methyl ethyl ketone) method was studied. An automated computer controlled module was developed based on the Dowex-1 separation methodology. The new chemical separation method recovered more than 80 % of 99mTc from the irradiated target. The recovered pertechnetate had requisite radionuclidic, radiochemical and chemical purity for the preparation of 99mTc-radiopharmaceuticals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
99mTc is a widely used radioisotope for the preparation of many radiopharmaceuticals in diagnostic nuclear medicine [1]. The radioisotope 99mTc is typically obtained from the 99Mo/99mTc generator made with a alumina column pre-adsorbed with 99Mo of high specific activity (HSA) obtained by thermal neutron fission of 235U [2–4]. Five old reactors (NRU of Canada, HFR of Netherlands, BR2 of Belgium, Safari-1 of South Africa and Osiris of France) meet almost entire global demand of HSA 99Mo. The interest to study the production of 99mTc by the direct route via the 100Mo(p, 2n)99mTc reaction in a cyclotron [5] as an alternate source of 99mTc started only a few years back when the nuclear medicine community faced the scarcity of 99mTc due to planned and unplanned shutdown of a couple of reactors (NRU, Canada; HFR-Patten, Netherlands). Research activities in this direction include the study of the excitation function of 99mTc and other isotopic and nonisotopic active/stable isotopes produced concurrently by proton induced reactions on Mo target [6–14], development of a target irradiation system which can utilize high beam current [15], development of a fast chemical separation method [16–18], recovery of 100Mo from the irradiated target [19] and assessment of the directly produced 99mTc for the preparation of radiopharmaceuticals [13, 16]. It may be noted that various isotopes of Nb, Zr and Y could be produced at trace level [7, 12, 14, 19] when enriched 100Mo is irradiated with proton beam. It is, therefore, a challenge to produce 99mTc from 100Mo in cyclotron of requisite quality for human usage.
The separation of pertechnetate (from the bulk molybdate) can be achieved using one of the many strategies (e.g. liquid–liquid extraction [20], ion-exchange chromatography [17], aqueous biphasic extraction chromatography, ABECTM [18, 21], electrochemical method [22]).
During the study on the retention/elution behaviour of pertechnetate and molybdate ions on a tiny column of strongly basic anion-exchanger (Dowex-1 × 8), the potential to trap preferentially the no-carrier-added (nca) 99mTcO4 − from sodium hydroxide solution on a tiny column of strongly basic anion-exchanger (Dowex-1 × 8) was recognized [23, 24]. In this approach, low specific activity (LSA) [99Mo]Na2MoO4 in NaOH solution was passed down a tiny Dowex-1–8 column, the eluted solution collected and preserved for reuse. 99mTc preferentially retained on the Dowex-1 column, was recovered by elution with various eluting agents like perchlorate, nitrate, iodide, tetra butyl ammonium bromide (TBAB) and purified by adopting a suitable method [17, 23]. The method of purification is dependent on the nature of the eluting agent used. When the eluting agent is sodium iodide the purification was can be done by passage through a bed of AgCl plus alumina to remove excess I− and to control any breakthrough of 99Mo, respectively [23]. In another approach when the solution of TBAB in an organic solvent is used as eluting agent the purification was can be done by passage through a bed of alumina to remove excess TBAB and organic solvent and to control any breakthrough of 99Mo [17]. It was reported earlier that dilute nitric acid is a good agent to elute quantitatively 99mTc from the strong anion exchanger resin [23]. It is found that dilute nitric is very suitable agent to elute 99mTc and the whole process—separation from the LSA [99Mo]Na2MoO4, elution of 99mTc and its purification could be automated [25]. A simpler, and inexpensive, procedure for direct separation of 99mTcO4 − from (NH4)2 [99/100Mo]MoO4 solution using a small column of a strongly basic anion-exchanger (Dowex 1 × 8), is therefore developed and reported here. This separation method uses a Dowex 1 column in tandem with a small alumina column and dilute nitric acid is used as an eluant to elute 99mTcO4 − from the Dowex 1 column. The separation and purification of 99mTc produced by the 100Mo(p, 2n)99mTc reaction has also been attempted using the well known solvent (methyl ethyl ketone) extraction method to compare the quality of 99mTc obtained in Dowex-1 column chromatography method.
In the present work the authors have studied the yield of 99mTc produced from the proton irradiation of a natural molybdenum metal target as a function of incident proton energy, developed a new method of separation of the Tc radioisotopes from the irradiated molybdenum (natural/enriched) target, assessed the quality of the 99mTcO4 − in general and its radionuclidic purity (RNP) in particular as a function of time after the chemical separation of 99mTc from proton irradiated 100Mo target. Present study also includes the identification of Nb, Y and Zr (produced in trace amount during the irradiation of 100Mo target with proton beam depending on the composition of enriched Mo-100 target and energy of proton beam [7, 12, 14, 19]) in the two Mo/Tc separation methods employed. Radiotracers of Nb, Y and Zr required for this simulation study was produced by proton irradiation of a Zr target.
Experimental
Materials
Reagents such as hydrochloric acid, nitric acid, ammonium carbonate, sodium hydroxide pellets, hydrogen peroxide etc., were of analytical grade and were procured from E. Merck, India. Mo foils and Cu monitor foils were purchased from H. Cross Co. NJ 07074, USA, and Material Research Corporation, New York, USA, respectively. Anion exchanger: Dowex-1 × 8 (Cl− form, 200–400 mesh), capacity 3–5 meq/g of dry the resin and natural Mo metal powder (<150 μm, 99.99 %) were purchased from Sigma Chemical Co. St. Louis, MO. 63174, USA. Aluminium oxide, active basic and acidic (100–200 mesh), Brockman grade-1 (Prabhat Chemicals, Mumbai, India) were used in preparing the purification columns. [99Mo]Na2MoO4 in 5 N NaOH (150 mg Mo/ml: 1.11–2.22 GBq/ml) used in optimizing the separation of 99mTc from 99Mo and the cold kits MDP (methylene diphosphonate) and MIBI (methoxyisobutyl isonitrile) were obtained from the Radiopharmaceutical Division, BARC and BRIT, Mumbai, India. Enriched 100Mo (99.805 %) was obtained from Isoflex, USA, San Francisco, CA 94129, USA and its isotopic composition is shown in Table 1. Zr metal was purchased from H. Cross Co. NJ 07074, USA. Paper chromatography (PC) strips (3 MM Chr, 20 mm width) were purchased from Whatman International Limited, England.
Target preparation
Two types of targets were irradiated. For irradiation at low beam intensity and for short duration, natural Mo foils (25 micron) in a stack (containing 4-7 foils, 10 mm × 10 mm, with an 8–10 micron thick natural Cu monitor foil placed just before the first Mo foil) were used. Circular (10 mm diameter) natMo and 100Mo metal pellet targets were used in the irradiations of longer duration and at higher beam intensities (up to 3 μA). These Mo pellets were prepared by pressing about 400–500 mg natural or enriched Mo metal powder in a dice plunger at 980 MPa pressure. Zr-metal pallet was prepared by pressing about 350 mg natural Zr metal powder in a dice plunger at 980 MPa pressure.
Target irradiation
All the irradiation experiments were carried out with 16/18/20 MeV protons using the cyclotron of Variable Energy Cyclotron Centre (VECC), Kolkata. The beam current was measured by placing a natural copper monitor foil (8-10 mg/cm2 thick) in front of the target. For beam current measurement the natCu(p, x)62,63,65Zn cross-section data recommended by International Atomic Energy Agency [26] were used. In case of Mo-foil stack irradiation, stacks containing 4–7 natMo foils (each 25 micron thick) were irradiated with a proton beam (energy window: 8-20 MeV, current: 10–250 nA) for 1–5 min. In order to irradiate the stacked foil targets at lower incident beam energy, 100, 200, 300, 400 μm, thick Mo foils were used in between the Cu monitor foil and the Mo stack, to degrade the proton energy from 20/18/16 MeV to a lower value. The actual “on target” beam energy falling on the individual foil in a stack was determined using the energy-range formula and tables [27]. The radioactivity in each Mo foil in the stack was assessed 1.5 h after the end of irradiation (EOI). natMo pellet (about 400 mg) targets irradiated at 1–3 μA, 16/18 MeV beam intensity for 1–6 h were used to standardize the chemical separation of the Tc-radionuclides from Mo and other co-produced non-isotopic impurities (e.g. radioisotopes of Nb, Zr or Y) and to study the recovery of Mo from the irradiated target. 100Mo metal pellets were irradiated at 18 MeV. RNP of the 99mTc obtained from the enriched 100Mo target was studied as a function of time.
For the preparation of Nb, Zr and Y radiotracers, Zr-metal pallet (1 mm thick, w = 0.301 g) was irradiated in the in-house cyclotron of the Variable Energy Cyclotron Centre (VECC) with 18 MeV, 1 μA proton beam for 22 h.
Gamma ray spectrometry
The radioactivity of samples was measured on a 30 cc HPGe detector coupled to an ORTEC 92× Spectrum master and a personal computer loaded with ORTEC Maestro II software. The detector used had 10 % efficiency relative to a 3″ × 3″ NaI(Tl) detector and an energy resolution (FWHM) of 1.74 keV at the 1332 keV γ-peak of 60Co. A standard 152Eu source was used for the energy as well as for the efficiency calibration. The radionuclides were identified by their characteristic photo peaks and half-lives. In order to avoid any interference of counts from the same gamma energy of another radioisotope, carefully selected characteristic gamma energies of the respective radioisotopes were used for the activity measurement. The gamma energies used for the radioactivity measurement of different radioisotopes are listed in Table 2. All the nuclear data of these radioisotopes used for calculation were taken from the available Ref. [28]. The radioactivity measurement of all the radioactive samples was carried out at a suitable distance (0.5–25 cm) from the detector keeping the dead time below 10 %. Total error in the yield was estimated in the standard way: the independent errors of the linearly contributing processes (beam current measurement-8 %, statistical error in the counting-4 % and uncertainty in the sample geometry-5 %) were summed quadratically and the square root of the sum was taken. Thus, the total error estimated in the yield was about 10.2 %.
Direct counting of foils irradiated for short duration
Radioactivity in the irradiated foils were measured about 1.5 h after the EOI in order to estimate the activity of the radioisotopes having shorter half-life (e.g. 94mTc having t½ 52 min). Typical assay live times were 300 s for these samples. The radioactivity in the same irradiated foils were also measured for a longer duration and at a closer distance from the detector at different time points post irradiation in order to obtain a better counting statistics of those radioisotopes having longer half-lives. It is known that if the decay of the radioisotope during the measurement time is ignored, it will result in inaccuracy in radioactivity measurement. It has been calculated that when the measurement time duration is significantly longer compared to the half-life of the radioisotope (i.e., when duration of assay live time is larger than about 3/100th of the half-life of the radioisotope being assayed) the error in the activity estimation is increased beyond 1 %. In such situation the actual activity at the start of measurement was calculated using the following formula:
where A SOC = activity of the sample counted at the start of counting, c = total count of the sample for time t L, λ = decay constant of the radioisotope counted, ε γ = detector efficiency for the particular gamma energy counted, I γ = gamma ray intensity of the characteristic gamma peak of the radioisotope counted t R = real time of counting, t L = live time of counting.
From the radioassay data of each foil that was irradiated for 5 min, yields of various radioisotopes were calculated. Then by adding the yield of a particular radioisotope in successive foils, the cumulative yield (in 5 min irradiation) of that radioisotope in the thick target was calculated as a function of incident proton energy. From these data thick target yield for 1, 3 or 6 h irradiation was calculated using the following formula:
where A t = activity at the end of 1, 3 or 6 h irradiation, A t′ = activity at the end of 5 min irradiation, λ = decay constant of the isotope under consideration, \( \phi \) = proton flux during the 1, 3 or 6 h irradiation, \( \phi^{{^{\prime } }} \) = proton flux during the 5 min irradiation.
Target dissolution
Irradiated Mo target
Irradiated target was dissolved following a reported method [19]. The irradiated target was taken in a conical flask and 3 ml H2O2 (30 % w/w, E. Merck, India, Purified) was added to it and the flask was heated (50–60 °C) in a water bath. When the target reacted completely with H2O2, the flask was brought to room temperature and 1 ml of 3 M ammonium carbonate was added drop wise to yield a clear solution.
Irradiated Zr target
Irradiated Zr target produced 92mNb (t1/2 = 10.2 days), 95Nb (t½ = 3.61 days), 95Zr (t½ = 64 days) and 88Y (t½ = 106.6 days) tracers. One day after the end of the irradiation the target was dissolved in mixture of 10 ml conc. HCl and 2 ml HF. 0.1 ml of that solution was further diluted with 5 ml 6 M HCl. This diluted stock solution containing the radiotracers of Nb, Y and Zr was used in the experiment.
Chemical separation of the Tc-radionuclides from the irradiated thick natMo target
Preparation of the basic alumina column (required in MEK separation)
5 g of dry basic alumina obtained commercially was used directly for making this column, 80 mm (H) × 14 mm (dia.).
Anion exchange resin column (7 mm × 1 mm) preparation
A slurry of 25 mg Dowex-1 × 8, Cl− resin in 2 ml water was taken in a syringe and pushed into a polypropylene tube (internal diameter 1 mm, the other end of the tube was packed with some glass wool) to make the resin column. Both the ends of the tube were fitted with miniature barbed polypropylene fittings. The column was preconditioned by passing 10 ml of normal saline solution followed by washing with 5 ml water.
Preparation of the acidic alumina column (required in Dowex-1-HNO3 acid separation)
A slurry of 1.5 g acidic alumina in 5 ml water was poured into a glass column, 12 mm (H) × 8 mm (dia.), containing a sintered disc at the bottom. Excess liquid was drained off with the help of a vacuum pump and the column was washed thoroughly with 10 ml saline. Both the ends of the column were closed with silicon rubber septa having a small hole at the centre for insertion of a needle. Care was taken to avoid entrapment of any air bubbles in the column bed. A liquid trap was connected in-line with the vacuum pump (purchased from Waters, Milford, USA) to prevent any accidental contamination of the pump.
Studies on Nb, Zr and Y contamination in the final 99mTc preparation
This particular experiment was carried out to study the path of Nb, Zr or Y isotopes in the two separation methods used. Nb, Zr or Y radioisotopes could be produced directly through various nuclear reactions on Mo isotopes or indirectly through the decay of a particular radioisotope produced through a primary reaction; the amount of which depends on the energy of proton as well as the enrichment level of 100Mo used. Moreover, the purest 100Mo will produce 97Nb (t1/2 = 74 min) through 100Mo (p, α) reaction.
For the present study, an aliquot of the diluted stock solution containing the Nb, Y and Zr radiotracers was mixed with the solution containing Mo (both natural Mo and 99Mo) and 99mTc. The resultant solution was used to separate Mo and Tc. Both the Mo and Tc fractions were checked for the presence of Nb, Zr and Y radioisotopes.
Separation by MEK solvent extraction method
The solution obtained after the dissolution of the irradiated target was thoroughly agitated with 5 ml MEK in a vortex mixer. After standing for few minutes, the two layers got separated. The upper organic layer containing the Tc radionuclides was collected with a pipette and passed through a basic alumina column to trap any Mo impurity present in the extracted organic layer. The aqueous layer containing the molybdenum target material was preserved for the recovery of molybdenum. Aliquots of the radioactive solution were taken from all the radioactive solutions handled before and after the separation for the estimation of separation efficiency using γ-ray spectrometry in an HPGe detector.
Separation by Dowex-1 resin and HNO3 method
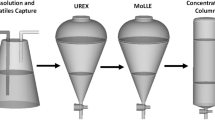
The flowchart of this separation procedure is shown in Fig. 1. In this method, at first the molybdate/pertechnetate solution was allowed to pass through the resin column with the help of a vacuum pump, which immobilized the pertechnetate and allowed the molybdate to flow. The molybdate solution was collected in the molybdenum collection vial. The Mo line and the resin column were washed with 1 ml of water and the washing was also collected in the molybdenum collection vial. The resin column was then washed with 5 ml of water and the washing was collected in the waste collection vial. 4 ml of 4 M HNO3 was passed through the resin column to elute 99mTc into the evaporation vial. The HNO3 line & the resin column were washed with 1 ml water and the washings were collected in the evaporation vial. The evaporation vial was then heated in a preheated temperature controlled oil bath (110 °C) while a flow of air was passed through the HNO3 acid line. HNO3 was thus evaporated out completely and the evaporated HNO3 was collected in an ice-cooled NaOH trap. The evaporation vial was then cooled and the requisite volume (10 ml) of saline was added. Sodium pertechnetate in saline thus obtained was passed through a small acidic alumina column (1.5 g), a Millipore filter (0.22 μm) and then collected in a vacuum vial. This method was tried using both molybdate/pertechnetate radioactivity obtained from cyclotron target and reactor production of 99Mo. The chemistry of separation and purification of 99mTc based on Dowex-HNO3 technique has been automated, named as TCM-AUTODOWNA (Figs. 2, 3), which utilizes abundantly available 99Mo(n,γ) produced by (n,γ) reaction in BARC reactors and also compatible for cyclotron produced 99mTc using enriched 100Mo. The automated system was controlled by a user friendly PC based graphical user interface that actually supervises the process via an embedded system based electronic controller [25, 29].
Labelling
MDP and MIBI labelling
2 ml of the radioactive [99mTc]pertechnetate solution obtained from the two separation methods was added separately to the freeze-dried kit vials of MDP (methylene diphosphonate) and MIBI (methoxyiobutyl isonitrile). The 99mTc-MDP preparation vials were kept at room temperature for 10 min while the 99mTc-MIBI preparation vials were kept in a boiling water bath for 10 min for the completion of the labelling reactions.
Quality assessment tests
The radioactive pertechnetate solutions obtained from the two separation methods were checked for the clarity, pH, radionuclidic purity (RNP) and radiochemical purity (RCP). The clarity of the solution was checked by visual inspection and pH was evaluated using a suitable pH indicating paper.
Radionuclidic purity (RNP) and radiochemical purity (RCP)
The RNP of the final product was estimated using a calibrated HPGe detector. The RCP of radioactive TcO4 − solution was evaluated by paper chromatography using Whatman paper strip (10 cm × 1 cm) and MEK as mobile phase. Radionuclidic purity due to the presence of other Tc radioisotopes in the final 99mTc preparation was checked in an HPGe detector as a function of time by counting an aliquot of decayed 99mTc sample. The RCP of 99mTc-MDP was evaluated by developing the Whatman paper strip (10 cm × 1 cm), spotted with the sample, in MEK solvent and saline. The radiochemical purity of 99mTc-MIBI was evaluated by developing the Whatman paper strip (10 cm × 1 cm), spotted with the sample, in MEK solvent and ethanol.
Chemical purity
MEK, molybdenum and aluminium contents in the final radioactive TcO4 − solution were determined by turbidity/color tests using iodoform, potassium thiocyanate and chromazural-S tests, respectively, as per the BRIT, Mumbai, India monograph.
MEK content test
200 μl of 1 N NaOH, 200 μl of 0.1 N I2, 50 μl of the test solution and 150 μl distilled water were taken in a test tube. The turbidity produced in the sample was compared with that of the standard (0.1 % v/v).
Molybdenum content test
50 μl of the test sample, 50 μl of 10 % potassium thiocyanate and 10 % SnCl2 were taken in a test tube. The orange-red colour produced in the sample was compared with that of the standard (10 ppm).
Aluminium content test
10 μl of the test sample, 30 μl of acetate buffer (0.1 M sodium acetate and 0.1 M acetic acid, pH = 4.6) and 10 μl of chromazural-S (2.7 mM) were taken in a test tube. The reddish pink colour produced in the sample was compared with that of the standard (10 ppm).
Nitrate (NO − 3 ) content test
The level of nitrate ion in the final radioactive TcO4 − solutions was measured using colorimetric test strips (Merck, Germany, Cat. No. 1.10020.0001). This test strips measure the nitrate ion concentration semi-quantitatively by visual comparison of the reaction zone of the test strip with the fields of a colour scale which can measure 10–500 mg/l of NO3 −.
Hydrogen Peroxide (H2O2) content test
Similarly, the presence of hydrogen peroxide in the final radioactive TcO4 − solutions was measured using colorimetric test strips (Merck, Germany, Cat. No. 1.10011.0001). This test strips measure the peroxide concentration semi-quantitatively by visual comparison of the reaction zone of the test strip with the fields of a colour scale which can measure 0.5–25 mg/l of peroxide.
Recovery of Mo from the irradiated target
The aqueous fraction containing ammonium molybdate obtained after the solvent extraction with MEK or the eluate obtained after passing the load solution through the resin column in the Dowex-1 resin and HNO3 method can be used to recover the enriched target material. In order to standardize the Mo recovery, 500 mg of Mo metal was dissolved as per the method described in “Target dissolution” section. To this solution 200 μCi of 99Mo was added. Then from this solution the 99mTc and 99Mo fractions were separated by the two separation methods described above. Total 99Mo activities in the solution before separation and in the Mo fraction obtained after separation were estimated. In the Mo fraction, molybdenum was present as ammonium molybdate. This fraction also contained some ammonium carbonate which was used during Mo metal dissolution step.
Results and discussion
Stacked foil irradiation: thick target yield of 99mTc
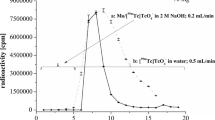
Radioactivity of various Tc radioisotopes produced in each foils in the stacks, irradiated for 5 min, was determined from the gamma spectrometry data. Then the yield of Tc radioisotopes in each foil for 5 min irradiation with 1 μA proton beam was calculated as a function of the incident proton energy (Fig. 4). From these data by adding up the radioactivity of the respective radioisotope in successive foils, the thick target yield (TTY) for 5 min irradiation with 1 μA proton beam was calculated and plotted as a function of the incident proton energy (Fig. 5).
It may be noted that the direct production of all these Tc radioisotopes (except 99mTc) can take place through more than one nuclear reaction channel. However, 99mTc can only be produced directly through 100Mo(p, 2n) reaction. For this reason the experimental TTY data obtained from this study can be directly extrapolated for enriched 100Mo target. The TTY of 99mTc thus calculated at the end of irradiation (EOI) for 100 % enriched 100Mo target for 1 h, 3 h, and 6 h irradiations with 100 μA proton beam is represented in Fig. 6 It is evident from this figure that if an 100 % enriched 100Mo target is irradiated at 20 → 8 MeV, 100 μA proton beam for 1 h, 3 h, and 6 h, it will produce about 60, 160, 272 GBq of 99mTc at EOI, respectively.
Chemical separation
Dowex-1 resin and HNO3 method
In the new Dowex-1 resin and HNO3 method, pertechnetate was bound efficiently (95 %) and eluted from the resin with very high yield (90 %). 99Mo along with the non-radioactive molybdenum target material ended up in the waste stream. The overall yield of 99mTc in both the separation methods was about 80 %.
Quality assessment of the purified 99mTc
A challenge in the development of cyclotron produced 99mTc is to ensure that the quality of the final pertechnetate preparation to be equivalent to that obtained from a 99Mo/99mTc generator.
At the end of the chemical separation, the product was evaluated for radionuclidic, radiochemical and chemical purity using the standard quality assessment protocols. The quality assessment results are summarized in Table 3. The radiochemical purity of the pertechnetate preparation was found to be 99 %. The efficacy in labelling of MDP and MIBI with the pertechnetate solution obtained from either of the separation methods was typically more than 95 %.
In both the separation processes no 99Mo was detected in the product vial containing radioactive TcO4 −. It was found from the simulation studies that 99.8 % Nb was removed in the washing process and 0.07 % of initial amount of Nb was present in the eluted 99mTc obtained in the Dowex method. Nb peak was absent in the organic fraction obtained in the MEK solvent extraction process. Level of Zr and Y present in the final radioactive 99mTcO4 − obtained by the solvent extraction method was below the detection level. In the Dowex separation method Zr level in the eluted 99mTc was 2.5 % of initial amount of Zr in the load solution. Level of Y in the eluted 99mTc obtained in the Dowex method was not estimated conclusively. Since the traces of Nb, Zr and Y radioisotopes that are produces in the irradiation of enriched 100Mo target are mostly collected in the Mo fraction after the Mo/Tc separation, they will not contaminate the product beyond acceptable level in either of the separation process.
MEK content in the final product was found to be less than 0.1 % (v/v). In both the methods of separation, nonradioactive molybdate content in the final pertechnetate solution was within the acceptable limit (less than 10 ppm). Al3+ levels were below the limits (less than 10 ppm) set for generator-produced pertechnetate. For both the processes, the pH of the final product solution was between 6 and 7. Paper chromatography showed that the pertechnetate obtained by processing of the targets irradiated in cyclotron was identical in radiochemical purity with the generator produced pertechnetate and no colloid was formed. The concentration of nitrate ions in the final pertecnetate solution was matching with 10 mg/l NO3 − colour zone in the test strip, which is much less than the LD50 value of nitrate (1267 mg/kg, oral-rat). The concentration of H2O2 in the final radioactive TcO4 − solutions was matching with 2–5 mg/l H2O2 colour zone, which is much less than the LD50 value of peroxide (1232 mg/kg, oral-rat).
Radionuclidic purity (RNP) of final pertechnatate solution prepared from the proton irradiated enriched 100Mo target was evaluated at different time points after its preparation and it was found that the RNP was greater than 99.9 % even at 24 h after the separation. The detailed analysis of level of different Tc radionulides is listed in Table 4.
Recovery of Mo from the irradiated target
Recovery of molybdate (ammonium molybdate) obtained in the two separation methods was estimated from the counting data and the recovery yield was found to be about 100 % in both the methods.
Conclusion
Present study of 99mTc yield in stacked foil irradiation shows that proton irradiation of enriched 100Mo target at 20 → 8 MeV, 100 μA proton beam for 1, 3, and 6 h, will produce about 60, 160 and 272 GBq of 99mTc at EOI, respectively. The new chemical separation method developed recovers more than 80 % of 99mTc from the irradiated target. The recovered pertechnetate has acceptable radionuclidic, radiochemical and chemical purity for labelling of biomolecules for clinical applications. The presented automated module is simple to operate and can be used to separate 99mTc radioisotope from low specific activity molybdenum.
References
Eckelman WC (2009) Unparalleled contribution of technetium-99m to medicine over 5 decades. JACC Cardiovasc Imaging 2:364–368
Boyd RE (1982) A molybdenum-99: technetium-99m generator. Radiochim Acta 30:123–145
Boyd RE (1987) Technetium generators: status and prospects. Radiochim Acta 41:59–63
Molinski VJ (1982) A review of 99mTc generator technology. Int J Appl Radiat Isot 33:811–819
Beaver J, Hupf H (1971) Production of 99mTc on a medical cyclotron: a feasibility study. J Nucl Med 12:739–741
Levkovskij V (1991) Activation cross section nuclides of average masses (A = 40–100) by protons and alpha-particles with average energies (E = 10–50 MeV). Data accessed from the NNDC EXFORdatabase, 2010. http://www.nndc.bnl.gov/exfor/
Lagunas-Solar MC, Kiefer PM, Carvacho OF, Lagunas CA, Cha YP (1991) Cyclotron production of nca 99mTc and 99Mo. An alternative non-reactor supply of instant 99mTc and 99Mo/99mTc generator. Appl Radiat Isot. 42:643–657
Scholten B, Lambrecht R, Cogneau M, Ruiz H, Qaim S (1999) Excitation functions for the cyclotron production of 99mTc and 99Mo. Appl Radiat Isot 51:69–80
Takács S, Szucs Z, Tárkányi F, Hermanne A, Sonck M (2003) Evaluation of proton induced reactions on Mo-100: new cross sections for production of Tc-99m and Mo-99. J Radioanal Nucl Chem 257:195–201
Challan M, Comsan M, Abou-Zeid M (2007) Thin target yields and EMPIRE-II predictions on the accelerator production of technetium-99m. Nucl Radiat Phys 2:1–12
Khandaker M, Uddin M, Kim K, Lee Y, Kim G (2007) Measurement of cross-sections for the (p, xn) reactions in natural molybdenum. Nucl Instrum Methods B 262:171–181
Lebeda O, Pruszyński M (2010) New measurement of excitation functions for (p, x) reactions on natMo with special regard to the formation of 95mTc, 96m+gTc, 99mTc and 99Mo. Appl Radiat Isot 68:2355–2365
Gagnon K, Bénard F, Kovacs M, Ruth TJ, Schaffer P, Wilson JS, McQuarrie SA (2011) Cyclotron production of 99mTc: experimental measurement of the 100Mo(p, x)99Mo, 99mTc and 99gTc excitation functions from 8 to18 MeV. Nucl Med Biol 38:907–916
Tárkányi F, Ditrói F, Hermanne A, Takács S, Ignatyuk AV (2012) Investigation of activation cross-sections of proton induced nuclear reactions on natMo up to 40 MeV: new data and evaluation. Nucl Instrum Methods B 280:45–73
Targholizadeh H, Raisali G, Jalilian AR, Rostampour N, Ensaf M, Dehghan MK (2010) Cyclotron production of technetium radionuclides using a natural metallic molybdenum thick target and consequent preparation of [Tc]-BRIDA as a radio-labelled kit sample. Nukleonika 55:113–118
Morley TJ, Dodd M, Gagnon K, Hanemaayer V, Wilson J, McQuarrie SA, English W, Ruth TJ, Bénard F, Schaffera P (2012) An automated module for the separation and purification of cyclotron-produced 99mTcO4 −. Nucl Med Biol 39:551–559
Chattopadhyay S, Das SS, Das MK, Goomer NC (2008) Recovery of 99mTc from Na2[99Mo]MoO4 solution obtained from reactor-produced (n, γ)99Mo using a tiny Dowex-1 column in tandem with a small alumina column. Appl Radiat Isot 66:1814–1817
McAlister DR, Horwitz EP (2009) Automated two column generator systems for medical radionuclides. Appl Radiat Isot 67:1985–1991
Gagnon K, Wilson JS, Holt CMB, Abrams DN, McEwan AJB, Mitlin D, McQuarrie SA (2012) Cyclotron production of 99mTc: recycling of enriched 100Mo metal targets. Appl Radiat Isot 70:1685–1690
Dallali N, Ghanbari M, Yamini Y, Fateh B, Agrawal YK (2007) Liquid-liquid extraction of ultra trace amounts of technetium produced by 100Mo (p, 2n) 99mTc nuclear reaction in cyclotron. Ind J Chem Sect A 46(10):1615–1617
Rogers RD, Zhang J (1996) Effects of increasing polymer hydrophobicity on distribution ratios of TcO4 − in polyethylene/poly(propylene glycol)-based aqueous biphasic systems. J Chromatogr B 680:231–236
Chakravarty R, Dash A, Venkatesh M (2010) A novel electrochemical technique for the production of clinical grade 99mTc using (n, γ) 99Mo. Nucl Med Biol 37:21–28
Chattopadhyay S, Das MK, Sarkar BR, Ramamoorthy N (2002) Separation of pertechnetate from molybdate by anion-exchange chromatography: recovery of 99mTc from (n, γ) 99Mo and suitability for use in central radiopharmacies (CRPh). Radiochim Acta 90:417–421
Huffman EH, Oswalt RL, Williams LA (1956) Anion-exchange separation of molybdenum and technetium and of tungsten and rhenium. J Inorg Nucl Chem 3(1):49–53
Chattopadhyay S, Das SS, Barua L, De A, Kumar U, Pal SS, Madhusmita, Neyar A Md, Das MK (2014) An automated computer controlled module for preparation of sodium [99mTc] pertechnetate using (n, γ) 99Mo-molybdate solution: TCM-AUTODOWNA generator. In: Proceedings at the 46th annual conference of Indian Society of nuclear medicine (SNMICON-2014), SINP, Kolkata, 11–14 Dec 2014. pp 26–27
IAEA (2009). http://www-nds.iaea.org/medical/monitor_reactions.html
Andersen HH, Ziegler JF (1977) Hydrogen stopping powers and ranges in all elements. Pergamon Press, New York
NuDat 2.6. http://www.nndc.bnl.gov/nudat2
Chattopadhyay S, Barua L, Das SS, De A, Kumar U, Mitra A, Mallick T, Madhusmita, Nayer MA, Sinha S, Sarkar BR, Ganguly S, De K, Das MK, Rajan MGR (2014) Pharmaceutical grade sodium [99mTc] pertechnetate from low specific activity 99Mo using an automated 99Mo/99mTc-TCM-autosolex generator. J Radioanal Nucl Chem 302:781–790
Acknowledgments
The work was performed in part under a research contract between the International Atomic Energy Agency (IAEA) and the Board of Radiation and Isotope Technology (BRIT), India (Research Contract No. 17159). The authors thank Dr. G Ganesh, Chief Executive, BRIT for encouragement. The authors are grateful to Dr. D.K. Srivastava, Director, VECC and cyclotron operators for target irradiations. Thanks are due to Target Laboratory of VECC for extending help in preparing thick Mo pellets from Mo powder.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, M.K., Madhusmita, Chattopadhyay, S. et al. Production and separation of 99mTc from cyclotron irradiated 100/naturalMo targets: a new automated module for separation of 99mTc from molybdenum targets. J Radioanal Nucl Chem 310, 423–432 (2016). https://doi.org/10.1007/s10967-016-4796-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4796-3