Abstract
We present an automated separation method to simultaneously isolate short-lived activation products 242g, 240Am, 237U, 72Ga from fresh fission products prior to gamma spectrometric analysis. On the basis of multi-column chromatographic units (HDEHP resin, TBP resin, DGA resin, TEVA resin, Al2O3, activated carbon) assembly, smart media compatibility between adjacent columns, chromatographic conditions optimization and process automation design, a modularized and streamline separation procedure was developed. The established method allows better recoveries of 93%, 91% and 97% for americium, uranium and gallium separately and excellent decontamination factors of more than 105 for fission products even for large volume of samples. Because of highly efficient removal of interferents, this method coupled with well-type high-purity germanium (HPGe) gamma spectrometers achieved highly sensitive and selective analysis of activation products in spiked complicated matrix samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is very interesting and challenging to quantitatively analyze activation products in freshly neutron-irradiated fissile materials by gamma spectrometry [1,2,3,4,5,6,7]. Americium, uranium and gallium are commonly existed in aged plutonium and uraniurm mixed oxides (MOX) fuel [8, 9]. Hence, the activation products of 242gAm, 240Am, 237U, 72Ga can be produced via nuclear reactions of (n,γ) and (n,2n). It is anticipated that the activity of each analyte is at the low level at the time of analysis due to short half-lives of 242gAm(16.02 h), 72Ga(14.1 h) [10] and lower nuclear reaction cross-sections of 241Am(n,2n)240Am [11] and 238U(n,2n)237U [12]. In view of its high detection efficiency, the well-type high-purity germanium (HPGe) gamma spectrometry is more suitable for analysis of these low activity gamma-emitting radionuclides than other types of HPGe detectors [13]. However, any nuclide to be measured by a HPGe detector must be radiochemically pure. Otherwise, the low-activity analyte can hardly be detected because of the serious signal shadowing under Compton background and coincidence summing effect resulting from a lot of gamma-emitting fission products (FPs). Additionally, in some cases, the amount of sample available for analysis is very limited. So, it is very necessary to simultaneously separate 242g, 240Am, 237U and 72Ga from a single sample. As a result of their short half-lives and low acitivities, a rapid and highly efficient separation and analytical method is urgently needed.
In the past few decades, a variety of chemical procedures have been reported to isolate 237U and/or Am isotopes from FPs applied to tracer preparation, radiochemical analysis, reaction cross-section measurement. These methods involved diethyl ether extraction, successive TBP extraction, HDEHP extraction, TIOA extraction, multi-step precipitations, cation and anion exchange, combined procedures [14,15,16,17,18,19,20,21,22,23]. However, extraction chromatography [24] offers similar selectivity as solvent extraction, high chromatographic utility, minimal utilization of organic reagents, and has been paid more attention by the researchers. Especially, Horwitz et al. has developed a great diversity of specific extraction resins [25]. Because of their good selectivity for specific radionuclide, these solid-phase extraction materials have been widely employed for chromatographic separation of U and Am isotopes from FPs and nuclear wastes prior to radiometric analysis. For example, the UTEVA resin-based extraction chromatography combined with anion exchange has been reported for rapid separation of 237U from mixed FPs followed by gamma spectrometric analysis [26]. Two stage anion exchanges (AG1 × 8) and two stage TEVA extraction chromatographies were reported to isolate short-lived 240Am from proton-irradiated bulk 242Pu target containing FPs for excitation function measurement of the 242Pu(p,3n)240Am reaction [27]. Multi-column extraction chromatography with TRU, TEVA and tertiary pyridine resins was established for analysis of long-lived 242mAm in low-level radioactive waste by β-ray spectrometry [28]. Nevertheless, these separation procedures based on the manual methodology are relatively troublesome and tedious involving complicated media conversion processes of evaporations by heating and redissolutions between columns or steps.
Instead, automation separation techniques [29, 30] have simplified and improved many analytical methodologies in comparison with mannual operations. Most of them focused on concentrating and separating long-lived Am and/or U isotopes from aged nuclear waste samples and environmental matrices prior to measurement by alpha spectrometry and/or mass spectrometry. Since the interference composition and measuring approach for the analytes are very different, these automated methods cannot directly meet the current requirements for detection of short-lived activation products 242g, 240Am, 237U and 72Ga among interferences of fresh FPs by gamma spectrometry. In our previous studies, an automated separation system coupled with off-line well-type HPGe gamma spectrometer has been developed for analysis of short-lived activation product 72Ga in the sample containing fresh FPs [31]. Due to the advantages of improved worker safety and rapid separation over traditional method, this system is very suited to the analysis of short-lived nuclides in the strong radiation samples. Based on its flexibility and modularization combined with the high selectivity of extraction resins described above, an attempt can be undertaken to extend our research to realize automated separation of multiple activation products 242g, 240Am, 237U and 72Ga from a single sample.
In this study, we investigated the systematic separation of Am, U and Ga from each other, and elucidated the behavior of Am and U on previous Ga separation module. Further purification of each analyte was performed by optimized column chromatographic conditions. Based on the multi-column extraction chromatographies and smart connections between columns, simultaneous separation of activation products Am, U, Ga from fresh FPs has been developed through the process of automation control. The separation performance was examined, and the proposed method was also applied to spiked complex matrices.
Experimental
Reagents, standards, and materials
The extraction chromatographic resins of N,N,N′,N′-tetra-(1-octyl)-3-oxapentane-1,5-diamide (DGA, normal), Aliquat 336 (TEVA) with grain sizes of 50–100 μm and 100–150 μm were purchased from Beijing UDLER Technology Co., Ltd (Beijing, China). The other solid phase extraction materials for column chromatography, such as resins of di-(2-ethylhexyl)-phosphoric acid (HDEHP) and tri-n-butyl phosphate (TBP), aluminum oxide (neutral) and activated carbon (AC), were employed as described previously [31]. The single element standard solutions of Na, K, Ca, Mg, Al, Fe, Ti, Mn (1000 μg mL−1) were purchased from the national standard material center (Beijing, China) and the standard used was made by combining these multiple single-element standards. L-ascorbic acid (abbreviated ACB), diethylenetriaminepentaacetic acid (DTPA) and lactic acid were from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Ultrapure water, with a resistivity of 18.2 MΩ⋅cm, was prepared using Milli-Q system. All other reagents used were of analytical grade.
The radionuclide 241Am was from China Isotope & Radiation Corporation (China). The 242gAm, 72Ga, and fission products were prepared by thermal neutron irradiation of 241Am, high-purity Ga2O3, and highly enriched uranium for an hour with a neutron flux of 1.0 × 1013 cm−2 s−1, respectively. The 237U was obtained by high-energy neutron irradiation of natural uranium for an hour followed by purification. These radionuclide solutions were supplied by Xi’an pulse reactor (Xi’an, China). The experiments were carried out with fission products cooled for three or four days after irradiation.
Apparatus
Gamma spectrometers equipped well-type HPGe detectors with an energy resolution of 2.30 keV for the 1.332 MeV 60Co peak to digital multi-channel analyzers were used for the measurement of the radioactivity of radionuclide in the solution.
Calibration of detection efficiency
The specific activity of the initial 242gAm, 237U, 72Ga solutions was standardized separately by a HPGe gamma activity standard apparatus. Its relative detection efficiency curve was traceable to 241Am, 133Ba, 137Cs, 152Eu, 60Co standards. According to the standardized 242gAm, 237U, 72Ga source solution, the absolute detection efficiency at principal γ-ray at 103 keV, 208 keV, 834 keV for a well-type HPGe detector was calibrated and proposed as 67.4 ± 1.1%, 26.2 ± 0.4%, 6.33 ± 0.09%, respectively.
Chromatographic separation
The dry resin or sorbent was filled into solid-phase extraction column (8.9 mm i.d. and 64 mm length, 5 mm i.d. and 220 mm length) by weighting, so as to effectively ensure the repeatability of separation. Before separation, 15 mL of a given concentration of reagent required was used to precondition the corresponding chromatographic column. Then, feed solution containing radionuclides was loaded onto the column. The elution separations were carried out with different eluents separately. The whole solution was driven by a peristaltic pump at desired flow rate. The radioactivity of nuclides in the fraction of the effluent was measured with a gamma spectrometer, and the elution profiles were drawn. The recovery for each analyte and the DF for each interfering radionuclide were calculated according to their radioactivity before and after separation.
Automated separation system
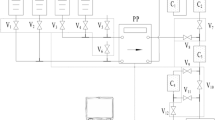
On the basis of our previous automated separation platform, the present system for Am, U and Ga was designed and developed. As shown in Fig. 1. The system mainly consists of five components: (1) Solvent reservoirs. 13 reservoirs (S1-13) were used for sample solution (S5) and reagents (S1, 3 mol L−1 HNO3; S2, 4 mol L−1 HCl; S3, 0.1 mol L−1 HNO3; S4, concentrated HCl; S6, 3 mol L−1 HCl-0.1 mol L−1 ACB; S7, 3 mol L−1 HCl; S8, 0.5 mol L−1 HNO3; S9, 3 mol L−1 HCl; S10, 0.1 mol L−1 HNO3; S11, 0.1 mol L−1 HCl; S12, 0.1 mol L−1 HNO3; S13, 0.05 mol L−1 Na5DTPA-1.0 mol L−1 Lactic acid (pH 3.0) (DTPA-Lac for short, the same as below) storage separately. Among them, the reagent S6 were used for rinsing, and the reagents S1-S4 (top), S7-S8 (middle), S9-S13 (bottom) were employed to further purify U, Ga, Am, respectively. (2) Solution collection vials. 4 vials were used to collect purified 242g, 240Am, 237U, 72Ga solution and liquid waste solution separately. (3) Peristaltic pump (PP). It was used to transfer the solution and reagents from solvent reservoirs to the subsequent chromatographic column units with a certain flow rate. (4) Chromatographic column units. 8 chromatographic columns (C1-C8) were employed in this system. They were packed with solid-phase materials such as HDEHP (C1), Al2O3 (C2), TBP (C3), TBP(C4), DGA (C5), mixed TEVA and DGA (C6), mixed Al2O3 and activated carbon (C7), HDEHP (C8). (5) Solenoid valves. Thirty-five solenoid valves (V2-5, V8-11 and V14-40) were used to control the selection of reservoirs and direction of liquid flow. The other five solenoid valves (V1, V6, V7, V12, V13) were employed to avoid vacuum formation in the pipeline because the S1-S13 containers are closed. The acid-resistant peristaltic pump tubes are employed to connect the corresponding components.
The controller and operational software of the present system are the same as previously used [31]. Based on the optimized separation conditions established, the required control parameters such as running time, flow rate and switch status of solenoid valves, were edited and entered into the operational software.
Separation procedure
As shown in Table 1, there are eight separation steps in the present automated system, which mainly involves column conditioning, sample loading, washing of interferences, and elution of each analyte.
A general separation procedure of 242g, 240Am, 237U, 72Ga with the related steps and the used parameters is descirbed in Table 2. As soon as the separation is started, the whole process will run automatically through the countdown of each step without human intervention. Additionally, the automated separation system can be reused after displacing S8, C1, C3 and its connecting tube with new one. The contamination between samples and memory effect can be eliminated by the next column conditioning steps. After separation, the radiochemically pure 242g, 240Am, 237U, 72Ga solutions collected were used for their activity analysis by off-line well-type HPGe detectors, respectively.
It can be seen that separation and purification of these nuclides are going on at the same time according to their individual modules during the running process of the system. The production solutions of Ga, U, Am were collected separately in sequence, and the total separation time including column conditioning was less than 3.0 h. Moreover, the system installation and replacement of disposable components can be accomplished within 20 min.
Results and discussion
Systematic separation considerations and column conditions optimization
Because fresh fission products contain plenty of gamma-emission interferents [32], automated separation and purification of low activity and short-lived activation products 242g, 240Am, 237U, 72Ga faces many difficulties and challenges. For instance, systematic separation of activation products from each other should be good enough to ensure effective recovery and mutual decontamination among them. And, high decontamination factors for fission products should be reached in each analyte fraction. Importantly, the separation process automation can be achieved only when the effluent from the former chromatographic column is suitable to serve as the load solution for subsequent columns. In this work, in order to maintain the continuity of our research, 3 M HCl was chosen as the initial feed solution media as before [31]. Tracer amounts of radioactive isotopes from real FPs and radionuclides of 241Am, 237U, 72Ga were employed to carry out the experimental studies and quantified by gamma spectrometry. Based on the extensive reports on extraction resins [24,25,26,27,28], we attempted to combine multiple chromatographic columns to solve systematic separation problem, smartly match the media compatibility between adjacent columns, and optimize further purification conditions for each analyte from interferences. So, it can be expected that the goal of automatic separation is to be reached.
Separation of Am, U, Ga from each other on several extraction resins
On the one hand, we considered whether a simple separation procedure could be designed for three analytes of Am(III), U(VI), Ga(III) independent of previous 72Ga separation procedure. According to the batch uptake data on TEVA resin reported by Horwitz et al. [33], the capacity factor (k′) for Am is below 0.1 over a wide range of HCl concentration, while k′ for U increases linearly with the increase of HCl concentration until k′ reaches 2500. Thus, we carried out the experiment of separating Am, U, Ga from each other by coupling TEVA resin with TBP resin. The elution behavior of Am, U and Ga on TEVA column was conducted, and the result was shown in Fig. 2a. It can be seen that Am can be separated from U and Ga, and recovered in the effluent after sample loading and cloumn rinsing with 3 M HCl due to its far poor sorption, whereas, the strongly retained U and Ga were rapidly stripped together with 20 mL of 3 M HNO3. The stripping solution of U and Ga can be directly introduced into the TBP column for their mutual separation. As shown in Fig. 2b, separation of Ga from U could be realized due to the fact that Ga was hardly retained on the TBP column while U was well retained. The above research indicates that the Am-U-Ga mixture in 3 M HCl can be separated from each other by tandem TEVA and TBP columns.
However, unlike Am and U, further purification of Ga from 3 M HNO3 media does suffer from a great limitation. For example, following TEVA and TBP column separations, there was still a certain amount of fission products such as 99Mo, 99mTc, 132Te, 131,132I and 103Ru in 3 M HNO3 solution containing Ga. Though this solution could be easily loaded onto a column of mixed Al2O3 and activated carbon for further purification, the decontamination of these interferents was not adequate for measurement requirements. Therefore, it is important to ensure that the solution media from systematic separation columns is not only suitable for being introduced into the subsequent column, but also convenient for further purification of each analyte.
On the other hand, we considered systematic separation of three analytes on the basis of previously established separation module of 72Ga. The elution behavior of Am and U on the related chromatographic columns was investigated. Similar to previous studies [24], the results also confirmed that U was strongly adsorbed on HDEHP column after column loading and rinsing with 3 M HCl, while Am was not retained on the successive HDEHP, Al2O3, TBP columns and obtained in the effluent. It can be concluded that systematic separation of Am, U, and Ga from each other has already been achieved by the previous 72Ga separation system. Then, various eluents (oxalic acid, ammonium carbonate, nitric acid, hydrochloric acid) were employed to strip U from the HDEHP column. The results suggested that even saturated H2C2O4 and a wide range of HNO3 concentrations were not capable of stripping U from the column, while 1.0 M NH4CO3 and high concentration HCl (10 ~ 12 M) were good enough to recover U. Nevertheless, it is not a good choice to employ (NH4)2CO3 as the eluent because additional media conversion is required for the subsequent purification. In the present study, for more efficiently stripping of U, concentrated HCl (12 M) was preferred as the literature report [34]. Therefore, we need to further purifiy Am and U from initial 3 M HCl and 12 M HCl solutions, respectively.
Purification of U on TBP resin and mixed TEVA and DGA resins
Our experimental results showed that though large amounts of FPs have been removed in the effluent of 3 M HCl from HDEHP column, minor fission products contaminated were still existed in the U stripping solution with 12 M HCl. The purity of U is insufficient for measurement requirement. Under the present column condition, U can be quantitatively retained on TBP resin using HCl solution with a concentration of higher than 6 M. So, the U stripping solution was directly introduced into TBP resin for retention and further purification. Figure 3 illustrated the elution behavior of U and fission products on TBP column. It can be seen that fission products of 99Mo, 99mTc, 132Te, 131,132I, 140Ba, 140La, 141,144Ce, 147Nd, 103Ru and 95Zr, 95Nb were eliminated by rinsing with 15 mL of 3 M HNO3 solution and 10 mL of 4 M HCl solution in sequence (Fig. 3a). Instead of stripping U with water or 0.01 M HNO3 as reported in the literatures [35, 36], 10 mL of 0.1 M HNO3 was used for quantitative desorption of U in our studies (Fig. 3b), which is well suitable for the further purification. It is also mentioned that though UTEVA resin [37] has stronger adsorption capacity for U than TBP resin, it was not used as stationary phase in the present column condition resulting from its incomplete recovery of U with pure dilute HNO3 (0.01–0.1 M) alone. Furthermore, after the U stripping solution of 0.1 M HNO3 was passed through mixed TEVA and DGA column, a very small amount of fission products (99mTc, 147Nd) were completely eliminated, while U was quickly recovered in the effluent without loss.
Purification of Am on DGA and HDEHP resins
DGA resin (normal) has the advantage of high uptake of trivalent actinides from high concentration hydrochloric acids [38]. Thus, following systematic separation of Am by tandem HDEHP, Al2O3 and TBP columns, the effluent of 3 M HCl solution containing Am was directly loaded onto the DGA column for further purification. The elution behavior of residual fission products and Am was illustrated in Fig. 4. As shown in Fig. 4a, major residual fission products of 140La, 103Ru, 140Ba, 131I and 91Sr was removed after column loading and rinsing with 3 M HCl. Subsequently, a portion of 99mTc, 141Ce, 140La, 140Ba and 91Sr was eliminated by washing with 0.1 M HNO3, which is conducive to the removal of U as well. However, because of their similar sorption capacity on DGA resin, major rare earth fission products of 147Nd, 144Ce and Am were fully recovered together in 10 mL of 0.1 M HCl stripping solution under the present experimental conditions (Fig. 4b), which is in good agreement with the literature [38].
Separation of trivalent actinides from lanthanides is very difficult owing to their similar chemical properties. Extraction chromatographic systems of HDEHP/DTPA-Lac [39] and Aliquat 336/NH4SCN-HCOOH [33] allowed good separation from each other. As a result of its low acidity, the stripping solution of Am from DGA column is well suitable to be introduced into the HDEHP column for subsequent purification. Following loading and rinsing with 0.1 M HCl and 0.1 M HNO3 in sequence, very few of contaminated divalent alkaline earth nuclides 140Ba, 91Sr and 99mTc, 103Ru, 132Te, 132I were further eliminated due to their far poor sorption on HDEHP column. Then, under optimized experimental conditions, Am was rapidly stripped from the column by employing 16 mL of complexing agent of 0.05 mol L−1 Na5DTPA-1.0 mol L−1 Lactic acid (DTPA-Lac) as our previous studies [40], whereas the lanthanides of 141Ce, 147Nd were removed due to their strong retention on the column.
Improvement on the purification and recovery of Ga
The elution conditions of Ga separation module were modified to enhance the decontamination for interferences and the recovery of analyte. On the one hand, by increasing the washing volume of 3 M HCl on TBP column from 25 to 35 mL, the decontamination factors (DFs) for some high-yield fission products such as 140Ba-140La, 141,144Ce, 147Nd and 132Te were significantly improved by at least one order of magnitude. On the other hand, after the stripping volume of 0.5 M HNO3 for Ga was increased from 4.5 mL to optimized 8 mL, the quantitative recovery of Ga was reached. Certainly, a little time is required to concentrate the production solution to an appropriate volume for measurement.
Separation scheme and process automation
Based on the above research, we proposed a chemical procedure for systematic separation of activation products 242g, 240Am, 237U and 72Ga from fission products. As illustrated in Fig. 5, on the basis of the previous Ga separation procedure (i.e. tandem HDEHP, Al2O3, TBP, mixed Al2O3 and activated carbon columns), the U separation procedure was developed by stripping U from HDEHP column and further purification with tandem TBP and mixed TEVA and DGA columns. Meanwhile, the Am separation procedure was exploited by introducing the effluent containing Am from coupled HDEHP, Al2O3, TBP columns into the DGA column. Based on the DGA column and HDEHP columns, the further purification of Am was performed. So, by means of multi-column partition and purification, high-purity 242g, 240Am, 237U and 72Ga can be obtained separately from a single sample solution. Although eight chromatographic columns were employed for separation and purification, all of the acidic media used for loading and stripping radionulcides between adjacent columns is well suitable for continuous separartion process without additional media conversion operations.
Benefiting from the continuous multi-column separation process established, an automated separation system for 242g, 240Am, 237U and 72Ga was developed based on simple valves and pump. As demonstrated in Fig. 1, the system has good-ordered layouts and mainly consists of clear functional modules (i.e. combined chromatographic unit) for separation and purification of three analytes. As can be seen, on the left, the tandem HDEHP resin (C1), TBP resin (C3), mixed TEVA and DGA resin (C6) columns serve as the module for 237U preparation. On the center, besides the HDEHP column, subsequent tandem Al2O3 (C2), TBP resin (C4), mixed Al2O3 and activated carbon (C7) columns serve as the module for 72Ga preparation. On the right, the tandem DGA resin (C5) and HDEHP resin (C8) columns serve as the module for 242g, 240Am preparation. The automated procedure (Table 1) was edited according to the optimum experimental conditions. Under program control, the separation process of sample solution loading (S5), columns rinsing (S6), reagents (S1-S4, S7-S8, S9-S13) transferring for elutions was performed automatically. Following sample loading and first rinsing, independent separation and purification was simultaneously carried out by each nuclide module.
Validation of the automated separation system
Several sets of experiments were carried out to test the validity of automated separation system established. We mixed a known radioactivity of 242gAm, 237U, 72Ga and thermal-neutron-induced fresh 235U fission products as the synthetic feed solutions, whose composition and content are similar to those expected in some specific samples at the time of analysis. The radioactivity of each analyte is at least two orders of magnitude lower than that of fission products. The characteristic peaks of 103 keV, 208 keV, 834 keV were selected as the analytical lines for 242gAm, 237U, 72Ga, respectively. As described previously, there is almost no interfering peak at 834 keV due to negligible fission background of 72Ga [31]. In addition, the background levels of 242gAm and 237U are negligible as well because none of them can be produced by thermal-neutron-irradiated 235U. The recovery and purity of the desired each radioactive analyte in the present system was examined by gamma spectrometry. Before separation, an aliquot of this feed solution was determined by gamma spectrometry. It indicated that the weak characteristic peaks of three analytes were completely overwhelmed by huge amounts of gamma rays from fresh fission products. After separation, a neat gamma spectrum for each analyte was appeared due to its high recovery and DFs for fission products. As listed in Table 3, the result shows that the present system allowed a high recovery of 93%, 91%, 97% for 242gAm, 237U, 72Ga, respectively, and DF of more than 105 for fission products. It is good enough to meet the accurate activity measurement. In addition, the present procedure is also well suitable to accurately determine short-lived gamma-emitting nuclide 240Am in some specific samples due to its consistent chemical behavior with 242gAm, and the characteristic peak of 988 keV can be chosen as the analytical line for gamma spectrometric analysis of 240Am.
The repeatability of recovery of the analyte and DF for interferences in the present system was examined. Since the HDEHP and TBP columns (C1 and C3) were in contact with concentrated HCl for elution, each column lifetime was greatly affected. Hence, we recommend replacing these two columns with new ones in time for the next use. In our first three test, the recoveries of Am, U, Ga with a RSD (n = 3) of 1.0% and DF of more than 105 for each fission product were achieved. Obviously, it is not required to trace the recovery of nuclides with their spikes or carriers due to the consistent repeatability. But in the fourth repetition, DFs will decrease to some extent as described previously [31]. So, it is proposed to replace all of columns after three repetitions in time. The reproducibility between columns by three tests also showed the accordant separating capability.
Application to the complicated matrix
The feasibility of the analytical method for complex matrix was evaluated. Because the certified reference materials similar to the present application cannot be available, the synthetic solution containing known specific activity of 242gAm, 237U, 72Ga, fresh fission products and complex components of 1 mg mL−1 Na, K, Ca, Mg, Al, Fe, Ti, Mn for each element, was prepared in 3 mol L−1 HCl-0.1 mol L−1 ACB media. It is noted that the reduction of ferric ions to the oxidation state + 2 with ascorbic acid is required. Assessment of this automation methodology was implemented by analyzing each 40 mL of the synthetic solution. The specific activity (Ac) of each activation product in sample solution was analyzed by the following formula:
where n is the detected radioactivity of analyte in production solution, cps. Pγ is γ-ray emission probability at 103 keV, 208 keV, 834 keV for each analyte, and its value is cited as 5.89%, 22.4%, 95.6%, respectively [3]. Ed is the detection efficiency at the principal energy of 103 keV, 208 keV, 834 keV, which was calibrated as 67.4%, 26.2%, 6.33%, respectively. Er is the chemical recovery of each analyte (Am, U, Ga) for the entire separation procedure, which was experimentally measured as 93%, 91%, 97%, respectively. k1 and k2 are the decay factors used to correct for the amount of short-lived analytes decay that occurs during the count interval and separation interval, respectively. Vs is the sample volume, mL.
The analytical results of specific activities (Ac) of the analytes are given in Table 4. The uncertainty of specific activity of 242gAm, 237U, 72Ga was evaluated as 1.5%, 1.4%, 1.3%, respectively, mainly from the uncertainty of relative detection efficiency curve for HPGe gamma activity standard apparatus. The uncertainty of each analytical result was synthesized based on Ed (1.6%, 1.5%, 1.4%) for 242gAm, 237U, 72Ga, respectively, and Er (1.0%), statistical error (1.0%) for each analyte. The analytical results were consistent with the standards added within relative deviation of ± 2.2%.
Conclusions
In summary, a novel automated separation method has been developed for systematic isolation of multiple short-lived activation products 242g, 240Am, 237U and 72Ga in neutron-irradiated fissile materials. Based on the idea of modularization and streamline separation, multi-column chromatographic units including HDEHP resin, TBP resin, DGA resin, TEVA resin, Al2O3, activated carbon were employed and the column chromatographic conditions were optimized. Moreover, cleverly choosing the loading and stripping conditions achieved well matching and compatibility of input–output media between adjacent columns. The established method presents a high recovery of over 90% for each analyte and high decontamination factors of more than 105 for fission products. Process automation control ensures fast separation (less than 3.0 h), better reproducibility and repeatability, reducing personnel cost and improving radiation safety for analysts. Due to high-efficient removal of interferents, the present method coupled with off-line well-type high-purity germanium (HPGe) gamma spectrometers realized highly selective and sensitive analysis of low-level activation products americium, uranium and gallium in spiked complicated matrix samples with a large volume.
This method may provide an attractive alternative to the existing procedures derived from traditional manual methodology. Based on our automation separation platform, future research will be devoted to the development of simultaneous separation and analysis of more variety of radionuclides interested and/or more complex samples, which will greatly improve the capability and efficiency of radiochemial analysis.
References
Douglas M, Friese JI, Greenwood LR, Farmer OT III, Thomas ML, Maiti TC, Finn EC, Garofoli SJ, Gassman PL, Huff MM, Schulte SM, Smith SC, Thomas KK, Bachelor PP (2009) Separation and quantification of chemically diverse analytes in neutron irradiated fissile materials. J Radioanal Nucl Chem 282:63–68
Morley SM, Seiner B, Finn E, Greenwood L, Smith SC, Gregory S, Haney M, Lucas D, Arrigo L, Beacham T, Swearingen K, Friese J, Douglas M, Metz L (2015) Integrated separation scheme for measuring a suite of fission and activation products from a fresh mixed fission and activation product sample. J Radioanal Nucl Chem 304:509–515
Morrison SS (2015) Activation product analysis in the presence of fission products. Washington State University, Pullman
Morrison SS, Seiner BN, Eggemeyer TA, Haney MM, Hines CC, King MD, Metz LA, Morley SM, Uhnak NE, Wall DE, Zhang ZC, Clark SB (2016) A chemical separation procedure using ionic liquid extraction for 59Fe and 55Fe quantification. J Radioanal Nucl Chem 307:2479–2485
Morrison SS, Clark SB, Eggemeyer TA, Finn EC, Hines CC, King MD, Metz LA, Morley SM, Snow MS, Wall DE, Seiner BN (2017) Activation product analysis in a mixed sample containing both fission and neutron activation products. J Radioanal Nucl Chem 314:2501–2506
Bennett KT, Kozimor SA, Manard BT, Mocko V, Pacheco SD, Schake AR, Wu R, Olson AC (2019) Rapid activation product separations from fission products and soil matrixes. J Radioanal Nucl Chem 322:281–289
McNamara BK, Morrison S, Pierson B (2021) Potential defect structure effects in the volatility separation of sub-picogram quantities of fission and activation products from an irradiated UO2 matrix. J Radioanal Nucl Chem 327:21–30
Rogozkin BD, Belov GV, Bergman GA (2009) Gallium removal during pyrochemical reprocessing of weapons plutonium into oxide fuel. At Energ 107:69–72
Saravanan T, Arunmuthu K, Lahiri BB, Bagavathiappan S, Venkiteswaran CN, Philip J, Divakar R, Joseph J, Jayakumar T (2015) Dimensional measurements on 112 GWd/t irradiated MOX fuel pins using X-ray radiography. Ann Nucl Energy 83:8–13
Firestone RB (1996) Table of isotopes, CD ROM edition, 8th edn. Wiley, New York
Kalamara A, Vlastou R, Kokkoris M (2016) Investigation of the 241Am(n,2n)240Am cross section. Phys Rev C 93:014610
Hashimoto T, Sotobayashi T, Kobayashi K (1978) Measurement of average cross section for 238U(n,2n)237U reaction. J Nucl Sci Technol 15:626–628
Gordon G, Hemingway JD (1995) Practical gamma-ray spectrometry [M]. Willey, England, p 48
Gosman A, Kliský V, Kašpar J, Vodolan P (1988) Preparation and application of 237U for the study of heterogeneous isotope exchange on an ion exchanger. J Radioanal Nucl Chem 121:375–383
Sabel’nikov AV, Maslov OD, Gustova MV, Belov AG, Dmitriev SN (2006) Preparation of 237 U by 238 U (γ, n) photonuclear reaction on an electron accelerator, MT-25 microtron. Radiochemistry 48:186–190
Maslov OD, Bozhikov GA, Ivanov PI, Gustova MV, Belov AG, Dmitriev SN (2010) Use of a nanostructured material for separation of 238U and 237U produced by the photonuclear reaction 238U(γ, n)237U. Radiochemistry 52(1):87–89
Moore FL, Reynolds SA (1959) Radiochemical determination of uranium-237. Anal Chem 31(6):1080–1081
Ueno K, Asano M, Kawasaki M (1971) Separation of uranium from fission products. J Nucl Sci Technol 8(3):129–132
Shinohara N, Hatsukawa Y, Hata K, Kohno N (1997) Radiochemical determination of neutron capture cross sections of 241Am. J Nucl Sci Technol 34(7):613–621
Felker LK, Benker DE, Chattin FR, Stacy RG (1995) Separation of americium, curium, and plutonium from irradiated targets. Sep Sci Technol 30(7–9):1769–1778
Ueno K, Watanabe K, Sagawa C, Ishimori T (1974) Separation of transplutonium elements from neutron irradiated americium-241. J Nucl Sci Technol 11(1):8–14
Kamoshida M, Fukasawa T (1996) Solvent extraction of americium (VI) by tri-n-butyl phosphate. J Nucl Sci Technol 33(5):403–408
Myasoedov VF, Molochnikova NP (1970) A procedure for concentrating americium and curium combined with their separation from plutonium and fission products using a chelating resin. J Radioanal Chem 6:67–73
Braun T, Ghersini G (1975) Extraction chromatography. Elsevier Scientific Publishing Company, Amsterdam
Bertelsen ER, Jackson JA, Shafer JC (2020) A survey of extraction chromatographic f-element separations developed by E. P. Horwitz. Solvent Extr Ion Exch 38:251–289
Haney MM, Seiner BN, Finn EC, Friese JI (2016) Rapid quantitation of uranium from mixed fission product samples. J Radioanal Nucl Chem 307:1737–1742
Ellison PA (2011) I.Nuclear production reaction and chemical isolation procedure for 240Am II.New superheavy element isotopes: 242Pu(48Ca,5n) 285114. Electronic Thesis and Dissertations, UC Berkeley
Shimada A, Kameo Y, Takahashi K (2013) A new method to analyze 242mAm in low-level radioactive waste based on extraction chromatography and β-ray spectrometry. Anal Chem 85:7726–7731
Cerdà V (2019) Automation of radiochemical analysis by flow techniques—a review, TrACTrends. Anal Chem 118:352–367
Qiao JX (2020) Dynamic flow approaches for automated radiochemical analysis in environmental, nuclear and medical applications. Molecules 25:1462–1488
Fan JL, Lu JC, Zhang LL, Yu GS, Huang P, Shi QL, Liu ZC, Li XS, Bai T (2016) Automated separation of short-lived 72Ga from fresh fission products based on tandem column chromatography. J Radioanal Nucl Chem 309(2):467–476
Dry DE, Bauer E, Petersen LA (2005) Rapid separation of fresh fission products. J Radioanal Nucl Chem 263(1):19–22
Horwitz EP, Dietz ML, Chiarizia R, DiamOnd H (1995) Separation and preconcentration of actinides by extraction chromatography using a supported liquid anion exchanger: application to the characterization of high-level nuclear waste solutions. Anal Chim Acta 310:63–78
Tomažič B, Siekierski S (1966) Separation of some fission products from uranium (VI) by reversed–phase partition chromatography. J Chromatog 21:98–104
Tamura N, Yonezawa C (1974) Extraction-chromatographic separation of uranium from long-lived fission products using tributyl phosphate. J Radioanal chem 20:455–462
Yamaura M, Matsuda HT (1997) Actinides and fission products extraction behavior in TBP/XAD7 chromatographic column. J Radioanal Nucl Chem 224(1–2):83–87
Horwitz EP, Dietz ML, Chiarizia R, Diamond H (1992) Separation and preconcentration of uranium from acidic media by extraction chromatography. Anal Chim Acta 266:25–37
Horwitz EP, McAlister DR, Bond AH, Barrans RE (2005) Novel extraction of chromatographic resins based on tetraalkyldiglycolamides: chracterization and potential applications. Solvent Extr IOn Exch 23:319–344
Kosyakov VN, Yerin EA (1978) Separation of transplutonium and rare-earth elements by extraction with HDEHP from DTPA solutions. J Radioanal Chem 43:37–51
Fan JL, Duan L, Wang YF, Zhang XB, Chen GW, Liang JF, Tian XJ, Li ZM (2022) Automated separation of Am from Sm by two-stage polymer-based HDEHP extraction chromatography. Colloids Surf A 654:130080–130089
Acknowledgements
We gratefully acknowledge Ning Yang from Xi’an pulse reactor (China) for preparation of the radionuclides.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, J., Wang, Y., Li, Z. et al. Automated multi-column chromatographic separation of short-lived 242g, 240Am, 237U and 72Ga from fresh fission products. J Radioanal Nucl Chem 332, 2667–2678 (2023). https://doi.org/10.1007/s10967-023-08920-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08920-6