Abstract
Quantitative measurement of fission and activation products resulting from neutron irradiation of fissile materials is of interest for applications in environmental monitoring, nuclear waste management, and national security. Based on established separation processes involving co-precipitation, solvent extraction, and ion-exchange and extraction chromatography, we have optimized a proposed sequence of separation steps to allow for the timely quantification of analytes of interest. We have recently evaluated this scheme using an irradiated sample to examine the adequacy of separations for measurement of desired analytes by gamma spectrometry. Here we present the radiochemical separations utilized and the yields and purity obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quantitative determination of a number of radionuclides in mixed fission and activation product samples (MFP/MAP) is needed to support applications such as nuclear data measurements, environmental monitoring, radioactive waste treatment, and nuclear safeguards [1]. While the resolution afforded by high-purity germanium (HPGe) gamma spectrometry is sufficient for the determination of several radionuclides in bulk samples, chemical separations are necessary to remove interferences and enable the measurement of others. In cases where sample size is limited, a sequential series of separations is necessary to maximize the probability of measuring analytes at low levels.
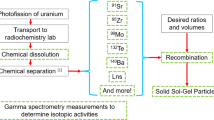
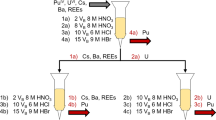
In this work, we developed three conceptualized approaches to the separation of MFP/MAP samples into fractions for the determination of gamma-emitting radionuclides by gamma spectrometry. Abbreviated flow diagrams of these separations, based on reported chemical behavior, are shown in Fig. 1a–c [2–11]. Evaluation and refinement of these separations with neat radiotracer solutions and more complex soil digestates has been performed. However, due in part to insufficient detection limits during elemental analysis by ICP-OES, such studies make it difficult to demonstrate that the yield and purity of the desired analyte in a given fraction will be sufficient to result in its measurement by gamma spectrometry. For example, a small quantity impurity in a separated fraction of a high yield fission product with a short half-life and numerous gamma emissions, such as 132I, may be sufficient to prevent the measurement of other low-yield or low-branching ratio gamma-emitting nuclides.
Here we describe an experiment with irradiated highly enriched uranium, containing the complete suite of fission products, which was then spiked with a consistent atom-quantity (nominally 1 × 109 atoms) of activation product nuclides which had been produced by (n, γ) reactions on stable metals. Replicate samples then underwent the three sequential separations processes displayed in Fig. 1, which were evaluated for their utility in isolating the low-level activation product radionuclides (Table 1). Determination of their yield, the extent to which other radionuclides were present, and ultimately whether the separations were adequate to permit their determination by gamma spectrometry were the primary objectives.
Experimental
Neutron irradiation was completed at the Dodgen Research Facility 1-MW TRIGA reactor operated by the Nuclear Radiation Center at Washington State University (Pullman, WA). The reactor has a nominal thermal neutron flux of 4 × 1012 neutrons/cm2 s. A highly enriched uranium metal foil (93% 235U) and seven stable metals (Table 1) of natural isotopic composition were doubly packaged in polyethylene vials and irradiated for sufficient time to achieve approximately 1 × 1014 fissions of 235U in the foil and 1 × 1012 atoms of each (n, γ) activation product radionuclide (when decay corrected to the end of irradiation, 1225 PDT, March 9, 2009). A flux monitor consisting of 5 high-purity metals or alloys of known mass was included in the irradiation to determine the total thermal, epithermal, and fast neutron fluence received by samples.
Following transport to Pacific Northwest National Laboratory (PNNL), stock solutions of each irradiated material were prepared by dissolving the HEU foil and activated metals in appropriate inorganic acids (nitric, hydrochloric, and hydrofluoric). Gravimetrically aliquoted portions of each were analyzed by gamma spectrometry to determine activity concentrations of each stock solution. Aliquots of each activation product solution were then added to the dissolved HEU foil to generate a solution containing 7.25 × 1011 fissions of 235U per gram and nominal concentration of 1 × 10−3 atoms/fission of each activation product.
Aliquots of the combined MFP/MAP solution equivalent to 1 × 1012 fissions of 235U and approximately 1 × 109 atoms (corrected to the end of irradiation) were then processed through the three conceptually developed flow charts depicted in Fig. 1, in quadruplicate for Flow Charts 1a–b and in duplicate for Flow Chart 1c. A listing of activation product radionuclides and quantities present in replicate samples processed is provided in Table 1. Separated fractions were counted by gamma spectrometry to identify and quantify all gamma emitting radionuclides present. We chemically yielded analytes based on the known quantities of activation products contained in the gravimetrically aliquoted stock solutions processed for each replicate sample and determined instances and quantities of fission product impurities.
While not reported here, aliquots of each eluted fraction were prepared for inductively coupled plasma—atomic emission spectroscopy (ICP-AES) analysis. Prior to processing, samples were spiked with known quantities of activation product elements to account for analyte losses during separation. Analyses were performed as confirmatory measurements on calculated chemical yields, and to develop methods for chemical yielding by ICP-AES for unknown samples.
Results and discussion
Separated fractions were collected and prepared to conform to calibrated counting geometries. Based on known quantities of activation product radionuclides in the initial solution aliquots, yields from chemical separations were determined. All isotopes detected by gamma spectrometry for a given fraction were tabulated and recorded. The yields of activation product radionuclides in their anticipated fractions and primary impurities present in each fraction are reported below.
Separations Flow Chart A
In Flow Chart A, the desired activation product-containing fractions are Au, Sc, Fe, Mn, and W (indicated in the shaded boxes of Fig. 1a). Recoveries of these elements in the respective fraction, based on gamma spectrometry measurement of the activation product in Table 1 for that element, are shown in Fig. 2. Additionally, the activated nuclides of elements Ga, Zn, and Co were measured in one of the collected fractions, so are also included in this plot. Further detail of the purity of each fraction is described below.
Average recovery of activation product elements (n = 4) using Flow Chart A (Fig. 1 a)
Gold was determined to be quantitatively present within the collected Au fraction. The fraction was relatively pure, the highest contaminant being 103Ru, indicating that approximately 15% of the total Ru partitioned to this fraction. Up to 1% of the 239Np and 3–4% of lanthanide fission products including 140La, 141Ce, and 147Nd were also measured. Except for Ru, these fission product elements have little retention on anion-exchange resin in 3 M nitric acid, and higher purity could likely be achieved by increasing the rinse volume prior to elution of gold using 10% thiourea solution.
Scandium was recovered in quantitative yield from the Eichrom TRU resin. Its retention on this resin is expected due to its similar chemical behavior to the trivalent lanthanide elements, which are known to be retained on TRU resin in 3 M HNO3. However, it is noteworthy that it did not elute from this resin with the lanthanides in 4 M HCl, and instead eluted with a rinse of 4 M HCl/0.1 M HF performed for removal of Th and Zr. The only other fission products present in significant quantity in the Sc-fraction were 95Zr and 97Zr, at approximately 80% of their total. Their presence was anticipated, and did not interfere with a high precision measurement of the 46Sc (3–4% RSD).
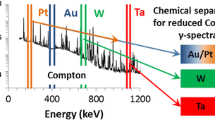
The 2-heptanone extraction of Fe proved to be quantitative for 59Fe recovery. It also extracted 78–95% of the 72Ga, 12–38% of 192Ir, and up to 78% of the 65Zn. Contaminant fission products found in this fraction included 132Te (75–80%), 95/97Zr (up to 15%), 127Sb (60–100%), and 103Ru (up to 6%). There are two high energy gamma emission peaks associated with 59Fe at 1099 and 1292 keV. While the peak at 1292 keV suffered interference from 132I (daughter of 132Te) and possibly 127Sb, there is no known interference with the 1099 keV emission, so measurement of this isotope was possible despite the presence of several other radionuclides.
54Mn was determined to be eluted quantitatively in the Mn fraction. This fraction also contained nearly 25% of the 192Ir; however, the uncertainty on the measurement was high. Fission product contaminants included 105Rh (~70% of the total) and 103Ru (35–47% of the total).
The W fraction had a low yield of 187W, an average value of 30% among the four replicate samples. Because several half-lives had transpired by the time samples were placed on the detector, counting uncertainties were relatively high, as indicated in Fig. 2 (30–32% RSD for each replicate). This fraction also contained 3–5% of the 54Mn, 7–14% of the 60Co, and 6–8% of the 192Ir. Fission product contaminants included 6–12% of the total 103Ru and 3% of the 105Rh.
The activation products 60Co and 65Zn were measured in significant yield in the Cd fraction, which was eluted after the W fraction. The 60Co recoveries ranged from 81 to 99% while those of 65Zn were 83–96%. The major fission product contaminants in this fraction included 103Ru (2–3% of the total) and 91Y (3% of the total).
Separations Flow Chart B
This conceptual separation scheme differs from that described above in that Eichrom resins are not used early in the sequence to remove the large mass of U. Instead, two co-precipitations are used in series to achieve group separations, followed by ion-exchange methods to isolate the activation products. The recoveries of the four activation product element fractions highlighted in Fig. 1b are presented in Fig. 3. Apart from Au, which was separated in the same fashion as in Flow Chart A, the recoveries of activation product radionuclides in the fractions anticipated were lower than desirable. While the recovery of 187W was improved over the previous flow chart, recoveries of 54Mn and 59Fe decreased. Further detail on the yield and purity of these fractions is provided below.
Average recovery of activation product elements (n = 4) using Flow Chart B (Fig. 1 b)
The Mn fraction displayed varying recoveries between the four replicates, from 16 to 59%, suggesting that some of the steps leading up to its isolation are sensitive to technique. The fraction also contained 4–7% of the 192Ir. No other activation product nuclides were measured. Impurity fission products measured in this fraction included 103Ru (1–2% of the total), 105Rh (7–10% of the total), 132Te (3–4% of the total), and 99Mo (4–6% of the total). Overall the Mn fraction had a relatively high purity, but improvement in the Mn recovery is desirable.
The W fraction had recoveries ranging from 41 to 56% based on measurement of 187W. From 1 to 3% of the 54Mn was also detected in this fraction; no other activation product nuclides were present. Once again, the fraction had a high degree of purity, with no fission products detected at levels above 1% of their initial value.
The 59Fe content in the four replicate samples was consistent, with recoveries ranging from 57 to 63%. A significant quantity of 132Te was also in the fraction (46–54% of the total), but no significant quantities (>2%) of other fission product nuclides were measured.
In the U fraction, 62–100% of the 60Co and 13–51% of the 192Ir were found. Major fission product radionuclides included 103Ru (43–69% of the total) and 69–91% of the 99Mo.
65Zn eluted in the Cd fraction, yielding 64–80% of the total in the 4 replicate samples.
In summary, this separation scheme achieved separated fractions of relatively high purity; however, further study is necessary to improve the chemical recoveries of activation product analytes.
Separations Flow Chart C
The percentage recoveries of analyte elements in the intended elution fractions are shown in Fig. 4. Most elements were recovered at moderate levels of at least 50%. Exceptions to this were Cr, W, Au, and Mn. Most separations in this scheme achieved a high degree of purity, with low levels of fission products in activation product fractions. Further detail on each fraction is provided below.
Average recovery of activation product elements (n = 2) using Flow Chart C (Fig. 1c)
The Ir fraction contained from 42 to 60% of the total 192Ir. No other activation product nuclides were measured. However, from 90 to 100% of the 91Y fission product was also present in this fraction, indicating that its expected partial retention on the Eichrom TRU resin did not occur. However, the presence of 91Y did not interfere with the 192Ir measurement. The Ir fraction also contained 8–9% of the 105Rh.
The W fraction contained only 9–10% of the total 187W. It is possible that a significant portion of the W was retained on the TRU resin under our conditions, accounting for some of the loss. In addition, 4–17% of the 192Ir was measured in this fraction, indicating that a portion of this element was retained on the anion resin in concentrated HCl, an unexpected result, suggesting larger rinse volumes are necessary. Fission product contaminants in this fraction included 103Ru (16–19% of the total), 105Rh (6–12% of the total), and 127Sb (14–16% of the total).
The Au was qualitatively observed to have high retention on the anion column following direct counts of the column; however, we experienced difficulty in eluting the Au using a hydrochloric acid gradient. To obtain a calibrated counting geometry, we muffled the resin and dissolved in acid. Unfortunately, during this process the Au was apparently volatilized, which accounts for the less than 1% yield shown in Fig. 4. Because the 10% thiourea solution effectively eluted Au in the other separation schemes, its use in this sequence will be investigated.
The Sc was retained on the TRU resin, as described for Flow Chart A. It was eluted from the column with the U using ammonium bisoxalate. Between 91 and 94% of the Sc was present in this fraction. The U yields varied from 59 to 87%. No further separation of Sc from U was pursued in this case, as acceptable counting statistics were obtained for the 46Sc analyte. The only fission product measured in this fraction in significant yield was 95/97Zr, at 6% of the total.
The Co fraction was collected during a nitric acid gradient elution by a CS5 high performance ion chromatograph. It contained from 69 to 79% of the 60Co, and no other activation or fission product nuclides were present at levels exceeding 2% of their total.
The Zn fraction contained 64% of the total 65Zn in each of the replicate samples. No other activation or fission products were measured in this fraction at levels exceeding 2% of their total.
The Ga fraction contained 77–80% of the 72Ga, from 3 to 6% of the 59Fe, and 4–10% of the 105Rh. No other fission product nuclides were measured at significant levels (>2%).
The Cr fraction had variable recovery in the two replicate samples, from 18 to 35%. While no other fission products or activation products were measured at levels exceeding 2% of their total, improved yield of Cr will be the subject of future work. Currently it is suspected that a significant portion of the Cr is partially retained on the TRU resin, and is co-eluted with Fe. However, measurement of Cr is difficult in the Fe fraction due to the presence of 105Rh, which has an interfering gamma emission at 320 keV.
In this experiment, the rinse of the TRU column was expected to contain a significant portion of the Fe, together with Mo and Y. While further separation could be performed (as indicated in Fig. 1c), in this case we elected to count the combined sample to determine whether 59Fe could be adequately measured in the presence of these other nuclides. We ascertained that 71–80% of the 59Fe was present in the fraction with 1-sigma counting uncertainties of 2–3%. Other radionuclides present in this fraction included 91Y (24–32%), 99Mo (54–74%), 54Mn (8–9%), 103Ru (6–7%), and 105Rh (3%).
Conclusions
In this work we evaluated three different sequences of separations for the measurement of a group of activation product radionuclides by gamma spectrometry in a mixed fission product/mixed activation product sample. While all activation product analytes were measured by at least one of the separations, improvements in yield and purity will be the focus of future work.
References
Dry DE, Bauer E, Petersen LA (2005) J Radioanal Nucl Chem 263:19
Maiti TC, Kaye JH (1995) J Radioanal Nucl Chem 190:175
Horwitz EP, Dietz ML, Fisher DE (1991) Anal Chem 63:522
Korkish J (1989) Handbook of ion exchange resins: their application to inorganic analytical chemistry, vol VI. CRC Press, Boca Raton, p 256
Weinert CHSW, Strelow FEW, Bohmer GR (1983) Talanta 30:413
Bunny LR, Ballou NE, Pascual J, Foti S (1959) Anal Chem 31:324
Kraus KA, Nelson F (1955) Proceedings of the international conference on the peaceful uses of atomic energy, Geneva, vol 7. p 113
Kraus KA, Nelson F, Moore GE (1955) J Am Chem Soc 77:3972
Nelson F, Kraus KA (1955) J Am Chem Soc 77:801
Kraus KA, Nelson F (1954) J Am Chem Soc 76:984
Kraus KA, Moore GE (1953) J Am Chem Soc 75:1460
Acknowledgements
We gratefully recognize funding by NA-22, Office of Nuclear Detonation Detection, Nonproliferation Research and Development, National Nuclear Security Administration, U.S. Department of Energy, and Director Randy Bell. We thank Don Wall of the Dodgen Research Facility and Nuclear Radiation Center at Washington State University for his assistance with the neutron irradiation of HEU and production of activation product radionuclides.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Douglas, M., Friese, J.I., Greenwood, L.R. et al. Separation and quantification of chemically diverse analytes in neutron irradiated fissile materials. J Radioanal Nucl Chem 282, 63–68 (2009). https://doi.org/10.1007/s10967-009-0263-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0263-8