Abstract
The provision of high quality spike isotopic reference materials is one of the objectives of the Joint Research Centre of the European Commission. They play an important part in measurements of nuclear materials for Nuclear Safeguards. Spike isotopic reference materials are prepared and certified according to the international standards. The assigned values for the isotope amount content and isotope ratios need to be verified at regular intervals. This is carried out by stability monitoring measurements. This paper will discuss the results of the stability assessment of some 242Pu and 243Am spike isotopic reference materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Confidence in comparability and reliability of measurement results in nuclear material analysis is established by production and dissemination of internationally accepted quality assurance tools such as certified reference materials (CRMs), reference measurements, and inter-laboratory comparisons (ILCs). They provide the basis for a strong verification system to safeguard nuclear activities in line with the international agreements [1, 2]. The fundamental role of CRMs in measurements is to establish traceability of a measured value to a primary unit of measurement, such as defined in the International System of Units (SI). Only measurement results that are traceable to a common reference, preferably the respective SI unit by unbroken chain of comparisons (i.e. traceability) can be regarded as truly comparable.
The Joint Research Centre of the European Commission in Geel (JRC-Geel) is a recognised producer of nuclear CRMs for Nuclear Safeguards and Nuclear Security [3]. They are part of a systematic programme to supply the international community with reference materials, in particular uranium and plutonium isotopic reference materials (IRMs) covering a wide range of concentrations and isotope ratios. IRMs are applied in mass spectrometry for calibration of instruments, in validation of analytical methods and for assessing the reproducibility and uncertainty of measurement results [4, 5]. One group of IRMs are the so-called “spike” reference materials applied in Isotope Dilution Mass Spectrometry (IDMS) for the determination of the amount content of nuclear materials [6,7,8,9]. Spikes are isotopically enriched materials with certified amount content and isotopic composition.
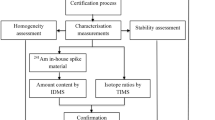
The preparation and certification of spike IRMs are demanding and challenging tasks that require state-of-the-art measurement procedures and equipment. For the last decade, the JRC-Geel has focused to align these procedures with the international standards such as the ISO 17034 and ISO Guide 35 [10,11,12]. A special emphasis was given to the assessment of the homogeneity and stability of spike reference materials and to include the corresponding uncertainty contributions in the final uncertainty of the reference material. This is important in view of providing fit-for-purpose and high quality IRMs with small uncertainties [13, 14]. IRMs are essential for laboratories striving to meet the international target values for measurement uncertainties in safeguarding nuclear materials (ITV2010) [15]. Various steps in the preparation and certification of IRMs are shown in Fig. 1.
As mentioned above, the stability of spike IRMs is an important aspect that needs to be addressed during the certification process. The term “stability” in this context refers to any changes that affect the assigned values of the reference material for the duration of the material’s shelf-time. The stability assessment is usually carried out in a dedicated experimental study over an extended period (e.g. 2 years) prior to the release of the reference material. Another way to establish stability behaviour is based on the experience with reference materials of similar characteristics (i.e. isotopic composition, concentration, and matrix). This data has been acquired at JRC-Geel over many years by participating in inter-laboratory comparisons (ILCs), between-lab verification campaigns and via in-house quality control. Some of the results have been published elsewhere [8, 16,17,18].
The behaviour of spike materials is difficult to predict reliably solely from stability studies over short periods. Evaporation over longer period can have an effect on the concentration.
For these reasons, spike IRMs, that have been produced more recently in compliance with the respective standards are subject to stability monitoring after release. Monitoring using the classical design is applied [12]. This involves the measurement of a number of units of a reference material at various points in time, for the duration of the material’s validity, as indicated on the reference material certificate.
Two 242Pu materials, namely IRMM-049d and IRMM-049e were selected for this study. 242Pu is commonly used as a spike in IDMS measurements for determination of the Pu content as this isotope is usually found only as a minor component in plutonium samples of the nuclear fuel cycle. Another selected material was the IRMM-0243 (referred to as STAM at CETAMA), which is a 243Am spike IRM used for applications in nuclear waste characterisation, nuclear forensics and nuclear decay studies [9]. These IRM solutions are supplied in glass ampoules with a screw-cap. They were introduced at JRC-Geel to replace the flame sealing of ampoules, which is no longer applied due to safety reasons. The results of the stability studies of these three spike IRM will be presented in this paper.
Experimental
Reference materials and test samples
For IDMS measurements, various IRMs for isotopes 239Pu, 240Pu and 244Pu were available at JRC-Geel. The following materials were chosen based on their availability, the certified isotope and their concentration.
-
IRMM-1027o and IRMM-1027m LSD: certified mass of 239Pu, 235U and 238U per vial. About 2 mg plutonium, (m(239Pu)/m(Pu) = 0.978) and about 50 mg uranium (m(235U)/m(U) = 0.194) as dried nitrate embedded in cellulose acetate butyrate (CAB)
-
IRMM-086: 239Pu solution, 3.7524 (22) µmol 239Pu g−1, m(239Pu)/m(Pu) = 0.978
-
IRMM-083: 240Pu solution, 3.4064 (22) µmol 240Pu g−1, m(240Pu)/m(Pu) = 0.990
-
IRMM-042a: 244Pu solution, 3.7507 (50) nmol 244Pu g−1, m(244Pu)/m(Pu) = 0.980
-
241Pu solution: 81.394 (53) nmol 241Pu g−1, m(241Pu)/m(Pu) = 0.993 [9, 19]
-
NBL 126 Pu: 239Pu solution, 7.0927(20) µmol 239Pu g−1, m(239Pu)/m(Pu) = 0.980, prepared gravimetrically from the 239Pu metal from the New Brunswick Laboratory Program Office (NBL PO) was used [17, 20].
In addition to the IRMs listed above, certified test items (with undisclosed values), EQRAIN and MOX-Pu4, were included in this study as part of the external quality control assessment. EQRAIN (Assessment of the Quality of Analysis Results in the Nuclear Industry) is the proficiency test for the analysis of 238U or 239Pu amount contents in nitrate solution organised at regular intervals by CEA/CETAMA [21, 22]. The JRC-Geel has participated in EQRAIN since 2008. MOX-Pu4 is a dried nitrate standard material prepared by the JAEA-PFDC (Japan Atomic Energy Agency, Plutonium Fuel and Development Centre).
An overview of IDMS measurements of IRMM-049d, IRMM-049e and IRMM-0243 materials using different IRMs and certified test items is shown in Fig. 2. Units analysed in the scope of the external quality assessment are highlighted in yellow.
Chemical treatment and isotopic measurements
Blends (e.g. spike + sample) for IDMS measurements were prepared by metrological weighing using the substitution method [17]. In most cases, more than one blend was prepared from each unit of the material being tested for stability. Prior to separation, an isotopic equilibration (homogenisation) of the spike and sample isotopes and the valence state adjustment were achieved by performing a reduction–oxidation step. The separations of the plutonium and americium samples were carried out by anion-exchange (Bio-rad AG®1 × 4, 100–200 mesh, Cl− form) and extraction chromatography (UTEVA, DGA), respectively. Details about the chemical separation procedures are published elsewhere [16, 18].
Isotopic measurements were performed by total evaporation (TE) method on a multi-collector Triton TIMS (Thermo Fisher Scientific, Bremen, Germany) [23,24,25,26,27]. In this method, the evaporation filament is heated up to maintain a steady intensity and isotopic ratios are measured until the entire sample is consumed (total evaporation). In this way, mass fractionation effects in the ion source are minimised. Isotopic standards (Pu IRMM-290b/A3, U IRMM-074/10) are measured to correct for these mass fractionation effects and to ensure the traceability to the International system of units (SI). A different approach was needed to correct for mass fractionation effects in Am measurements and is dealt with in "Results and Discussion" section.
The amount content was determined using the following IDMS equation [25]
cx is the element amount content of the "unknown" sample [mol g−1], cy is the element amount content of the spike [mol g−1], mx is the mass of the sample [g], my that of the spike [g], and Rx, Ry and Rb are the isotope amount ratios of the sample (unspiked) [mol mol−1], the spike and the blend, respectively. \(\Sigma {{(R}_{i})}_{x}\) and \(\Sigma {{(R}_{i})}_{y}\) are the sums of all isotope amount ratios in the sample and spike, respectively.
Uncertainties associated with IDMS measurements were estimated according to the Guide to Expression of Uncertainty in Measurement (GUM) using the GUM Workbench©, a software developed by Metrodata GmbH (Germany) [13, 14]. All the uncertainties in this paper are expanded uncertainties with a coverage factor k = 2, corresponding to a level of confidence of about 95%.
Results and discussion
IRMM-049d
IRMM-049d is a highly enriched 242Pu spike material (94.6%) certified for 242Pu isotope amount content and Pu isotope amount ratios. 93 units of this material were produced in 2011. Each unit contains about 10 mL of 5 M nitric acid in screw-cap glass ampoules with the plutonium mass fraction of approximately 0.1 mg Pu g−1 solution [28, 29].
The results of the IDMS measurements of the 242Pu amount content in IRMM-049d are shown in Figs. 3 and 4. Each data point represents an independent measurement result of the selected unit of IRMM-049d (spiking/weighing, chemical separation and replicate measurements).
IDMS results of the 242Pu amount content (mol g−1) measurements in the selected units of IRMM-049d using various spike materials: Eqrain (239Pu)—green squares (filled green square), IRMM-1027m (239Pu)—empty circles (open circle), 241Pu—brown circles (filled orange circle), JAEA MOX (239Pu)—purple triangles (filled purple triangle) and IRMM-042a (244Pu)—blue triangles (filled blue triangle). Error bars show the expanded uncertainty (k = 2) of the measured result. The red line shows the certified value and the red dashed line the expanded uncertainty (k = 2) of the certified value. (Color figure online)
The IDMS results obtained using different spike materials were in a good agreement with the certified value of the IRMM-049d. The average of all the measurements was 3.6676 (41) × 10−7 mol g−1 with a relative difference of + 0.023% from the certified value of 3.6668 (18) × 10−7 mol g−1. This small difference is within the uncertainty of the certified value (0.050%, k = 2). It can be concluded that there was no significant unit-to-unit variability observed in the measured units of IRMM-049d. Figure 4 also demonstrates that there was no significant change in the concentration of IRMM-049d over time.
IRMM-049e
As the IRMM-049d was approaching exhaustion, a new batch of this spike material was prepared in 2017 to ensure the continuous supply of the 242Pu spike material. The same 242Pu source solution was used as for its predecessor (IRMM-049d). 89 units of IRMM-049e material were made available with a similar concentration (i.e. ca. 1 mg Pu in 10 mL, 5 M HNO3) [18].
This batch of 242Pu spike IRM has been included in the stability monitoring after the material certification and release in 2017. The stability measurements were carried out for the amount content of plutonium and for the plutonium isotope amount ratios. This spike material was also included in various external EQRAIN campaigns (see Fig. 2). The results of the IDMS measurements of the 242Pu amount content in IRMM-049e are shown in Figs. 5 and 6. As for Figs. 3 and 4. each data point represents an independent measurement result.
IDMS results of the 242Pu amount content (mol g−1) measurements in the selected units of IRMM-049e using various spike materials: Eqrain (239Pu)—green squares (filled green square), IRMM-1027m (239Pu)—empty circles (open circle), IRMM-086 (239Pu)—yellow circles (filled yellow circle), NBL 126 (239Pu)—black squares (filled square) and IRMM-083 (240Pu)—empty triangles (open triangle). Error bars show the expanded uncertainty (k = 2) of the measured result. The red line shows the certified value and the red dashed line the expanded uncertainty (k = 2) of the certified value. (Color figure online)
It can be seen from Fig. 5 that the certified value for the 242Pu amount content in IRMM-049e was successfully verified using the selected spike materials. The average of all the measured results (22 units) was 3.5836 (46) × 10−7 mol g−1 with a relative difference of − 0.022% from the certified value of 3.5844 (45) × 10−7 mol g−1. Figure 6 shows that there was no significant change in the concentration of IRMM-049e over time.
The evaluation of the results of stability monitoring is also done by calculating the compatibility score (En) using the equation below.
This approach involves a comparison of each measured result (xmon) with the certified value (xCRM) and takes into account the standard uncertainties (umon, uCRM) and an appropriate coverage factor. A coverage factor k = 2 is chosen representing a level of confidence of approximately 95%. An absolute value of En less than 2 means that there is no significant difference between the two values, xmon and xCRM.
The compatibility scores in IRMM-049d and IRMM-049e are shown in Fig. 7. For all the results, a compatibility score (absolute value) smaller than 2 was obtained.
Two units of IRMM-049e were measured (#26 and #61) by TIMS for the determination of the Pu isotope amount ratios. The results of these measurements (October 22, 2020) are shown in Table 1.
All the results for the isotope ratios were in a good agreement with the certified value within measurement uncertainties, as well as highlighted by the compatibility scores (all |En |< 2).
IRMM-0243
IRMM-0243 is an Am spike IRM, certified for the amount content of 243Am and the n(241Am)/n(243Am) isotope amount. An americium source solution with an isotopic composition of 88% 243Am and 12% 241Am was used for the preparation of this material. 587 units were produced in cooperation with CEA/CETAMA. The concentration of the americium is about 1.5 μg mL−1 in 1 M nitric acid solution [9, 19]. This IRM was commercially released in 2017.
Two units of IRMM-0243 were selected (#197 and #313) for the measurement of the 243Am amount content and the Am isotopic composition by IDMS and TIMS, respectively. A 241Am spike, produced by ingrowth from a highly enriched 241Pu material (99.3%) was used as a spike for IDMS measurements. Isotope ratio measurements were carried out on the Triton TIMS using the total evaporation (TE) method. In the absence of a suitable Am isotopic standard at JRC-Geel, it was not possible to perform a correction for mass fractionation as is normally done for plutonium and uranium. Due to similarities in chemical behaviour and ionization energies, it can be assumed that Am behaves similarly to U or Pu during the total evaporation measurement and that the bias statements for U and Pu can be applied for Am [25]. For the n(243Am)/n(241Am) ratio, the maximum expected bias of 0.033% (k = 2, for ratios spanning 2 mass units) was applied. In addition, an uncertainty of 0.020% (k = 2, for ratios spanning 2 mass units) derived from isotope measurements of the U and Pu quality control (QC) materials was added as a conservative contribution to the uncertainty budget for Am [9, 19]. These two uncertainty components were included in the combined uncertainty for Am isotope measurements.
In the context of certification in 2017, one unit (# 3) was measured for isotope ratios only. The results of the 243Am amount content and isotope ratios in the IRMM-0243 stability monitoring study are shown in Tables 2 and 3, respectively.
The results of the 243Am amount content and the n(241Am)/n(243Am) amount ratio were in agreement with the certified values within uncertainties (all |En |< 2).
Conclusion
Measurements of the IRMM-049d, IRMM-049e and IRMM-0243/STAM spike IRMs have demonstrated that there were no stability issues associated with the assigned values. This was confirmed by the compatibility scores. Based on the results and the number of tested units, we can conclude that there was no significant change in the concentration of the three spike IRMs over time (up to 8 years) that could be due to evaporation of the solution in screw-cap ampoules. Furthermore, the results obtained in the scope of various proficiency testing rounds underpin the confidence in the assigned values and associated uncertainties. As a spin-off, the spike IRMs used to investigate the long-term stability are via this study interlinked with one another and indirectly their certified or assigned values are also mutually verified.
References
INFCIRC/153 (1972) The structure and content of agreements between the agency and states required in connection with the treaty on non-proliferation of nuclear weapons, INFCIRC/153 (corrected), Vienna
European Atomic Energy Community (2012) Consolidated version of the treaty establishing the European Atomic Energy Community. Off J Eur Union. 2012/C 327/01
Joint Research Centre, Certified Reference Materials catalogue. https://crm.jrc.ec.europa.eu/
Jakopič R, Sturm M, Kraiem M, Richter S, Aregbe Y (2013) Certified reference materials and reference methods for nuclear safeguards and security. J Environ Radioact 125:17–22
Mayer K, Wellum R (2004) Reference materials for destructive analysis in nuclear safeguards. ESARDA BULLETIN, No. 32 March
Vogl J, Pritzkow W (2010) Isotope dilution mass spectrometry—A primary method of measurement and its role for RM certification. J Metrol Soc India 25(3):135–164
Milton MJT, Quinn TJ (2001) Primary methods of the measurement of amount of substance. Metrologia 38:289–296
Jakopic R, Aregbe Y, Richter S, Zuleger E, Mialle S, Balsley SD, Repinc U, Hiess J (2017) Verification measurements of the IRMM-1027 and the IAEA large-sized dried (LSD) spikes. J Radioanal Nucl Chem 311:1781–1791
Jakopič R, Fankhauser A, Aregbe Y, Richter S, Crozet M, Maillard C, Rivier C, Roudil D, Marouli M, Tzika F, Altzitzoglou T, Pommé S (2021) 243Am certified reference material for mass spectrometry. J Radioanal Nucl Chem 327:495–504
ISO/IEC 17025:2005 (2005) General requirements for the competence of testing and calibration laboratories. International Organisation for Standardisation, Geneva
ISO/IEC 17034:2016 (2016) General requirements for the competence of reference material producers. International Organisation for Standardisation, Geneva
ISO Guide 35:2017 (2017) Reference materials—General and statistical principles for certification. International Organisation for Standardisation, Geneva
ISO/IEC Guide 98-3:2008 (2008) Evaluation of measurement uncertainty—Guide to the expression of uncertainty in measurement (GUM). International Organisation for Standardisation, Geneva
GUM Workbench Pro Version 2.4.1.458 1996-2016 (2009) Software Package for the Evaluation of Uncertainty. Metrodata GmbH, Braunschweig, Germany. http://www.metrodata.de
IAEA-STR-368 (2010) International Target Values 2010 for Measurement Uncertainties in Safeguarding Nuclear Materials; Vienna. https://inis.iaea.org/collection/NCLCollectionStore/_Public/49/057/49057994.pdf
Jakopic R, Verbruggen A, Eykens R, Kehoe F, Kuhn H, Kushigeta Y, Jacobsson U, Bauwens J, Richter S, Wellum R, Aregbe Y (2010) An inter-calibration campaign using various selected Pu spike isotopic reference materials. J Radioanal Nucl Chem 286:449–454
Venchiarutti C, Jakopič R, Hennessy C, Toth K (2021) Preparation and characterisation of uranium and plutonium quality control samples for isotope dilution mass spectrometry measurements and uncertainty estimation. J Radioanal Nucl Chem 327:1305–1316
Venchiarutti C, Jakopic R, Richter S, Jacobsson U, Hennessy C, Aregbe Y (2017) Preparation and certification of a new batch of 242Pu spike: IRMM-049e, EUR 28747 EN
Jakopič R, Fankhauser A, Aregbe Y, Crozet M, Maillard C, Richter S, Rivier C, Roudil D, Altzitzoglou T, Pommé S, Marouli M, Tzika F (2017) Preparation and certification of a 243Am spike reference material: IRMM-0243, EUR 28748 EN
New Brunswick Laboratory Program Office, National Nuclear Security Administration. https://www.energy.gov/nnsa/nbl-program-office
CEA Marcoule, Commission d’ETAblissement des Méthodes d’Analyse. https://cetama.partenaires.cea.fr/
Crozet M, Roudil D, Rigaux C, Bertorello C, Picart S, Maillard C (2019) EQRAIN: uranium and plutonium interlaboratory exercises from 1997 to 2016 - comparison to ITVs-2010. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-018-6399-7
Richter S, Goldberg SA (2003) Improved techniques for high accuracy isotope ratio measurements of nuclear materials using thermal ionisation mass spectrometry. Int J Mass Spectrom 229:181–197
Callis EL, Abernathey RM (1991) High precision isotopic analysis of uranium and plutonium by total sample volatilization and signal integration. Int J Mass Spectrom Ion Process. https://doi.org/10.1016/0168-1176(91)80081-W
C1672–17: Standard test method for determination of uranium and plutonium isotopic composition or concentration by total evaporation method using a thermal ionisation mass spectrometer, ASTM International. https://webstore.ansi.org/Standards/ASTM/ASTMC167217
Boulyga S, Kappel SK, Richter S, Sangely L (2015) Mass spectrometric analysis for nuclear safeguards. J Anal At Spectrom 30:1469–1489
Bürger S, Balsley SD, Baumann SD, Berger J, Boulyga SF, Cunningham JA, Kappel S, Koepf A, Poths J (2012) Uranium and plutonium analysis of nuclear material samples by multi-collector thermal ionization mass spectrometry: quality control, measurement uncertainty, and metrological traceability. Int J Mass Spectrom 311:40–50
Jakopic R, Bauwens J, Richter S, Sturm M, Verbruggen A, Wellum R, Eykens R, Kehoe F, Kuhn H, Aregbe Y (2011) Preparation and development of new Pu spike isotopic reference materials at IRMM. ESARDA Bull 46:65
Jakopic R, Eykens R, Kehoe F, Kuhn H, Richter S, Verbruggen A, Aregbe Y (2011) Preparation and certification of the isotopic reference material IRMM-049d, EUR 24915 EN
Acknowledgements
The authors would like to thank Roger Wellum for his valuable comments on the paper. The authors would like to thank Ulf Jacobsson and Stephan Richter for performing and supervising mass spectrometry measurements. The authors are grateful to Renata Bujak for her support in the chemical purification and mass spectrometry measurements on the IRMM-0243 carried out in 2017 and to Adelheid Fankhauser for her work on the IRMM-0243 project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jakopič, R., Venchiarutti, C., Hennessy, C. et al. Stability monitoring of selected spike isotopic reference materials for isotope dilution mass spectrometry. J Radioanal Nucl Chem 331, 2175–2183 (2022). https://doi.org/10.1007/s10967-022-08271-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08271-8