Abstract
Isotope dilution mass spectrometry (IDMS) is an analytical technique capable of providing accurate and precise quantitation of trace isotope abundance and assay providing measurement uncertainties below 1 %. To achieve these low uncertainties, the IDMS method ideally utilizes chemically pure “spike” solutions that consist of a single highly enriched isotope that is well-characterized relating to the abundance of companion isotopes and concentration in solution. To address a current demand for accurate 137Cs/137Ba ratio measurements for “age” determination of radioactive 137Cs sources, Idaho National Laboratory (INL) is producing enriched 134Ba isotopes that are tobe used for IDMS spikes to accurately determine 137Ba accumulation from the decay of 137Cs. The final objective of this work it to provide a homogenous set of reference materials that the National Institute of Standards and Technology can certify as standard reference materials used for IDMS. The process that was developed at INL for the separation and isolation of Ba isotopes, chemical purification of the isotopes in solution, and the encapsulation of the materials will be described.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Isotope dilution mass spectrometry (IDMS) methods are extremely useful for obtaining trace elemental concentrations in unknown samples and for characterizing reference materials with very high accuracy and low uncertainty [1]. Briefly explained, isotope ratio measurements are used to determine elemental or isotope concentrations by adding to a known amount of unknown sample a known quantity of an isotopically enriched tracer (spike) solution that is well-characterized. Ideally, isotopes with low natural abundances that are enriched to more than 90 % (at.%) are desired for IDMS measurements because the neighboring isotopes in the spike will not contribute greatly to the uncertainty in the unknown solution analysis. It is possible with proper spike solutions to obtain elemental measurements of unknown species with uncertainties below 1 % if the spike is well characterized and has stated uncertainties. For the analysis of Ba, there are 134Ba spike solutions available from Isoflex USA and Oak Ridge National Laboratory Isotopes Division, but their products are enriched to 88.1 ± 0.4 and 84 %, respectively, and have 12–16 % concentrations of 135Ba, 136Ba, 137Ba, and 138Ba that have nominally stated abundances [2].
To address the need for more isotopically enriched reference materials, Idaho National Laboratory (INL) is currently operating a 90° sector 1.5 m radius electromagnetic isotope separator that is capable of separating all of the natural Ba isotopes to levels at or above 99 % purity [2]. Producing isotopically enriched solutions from the separated isotopes is difficult however, due to the ease with which samples can be contaminated with natural Ba during the process of harvesting and processing the enriched isotopes. Furthermore, Ba isotopes are often collected onto metallic substrates such as high purity Al or Cu. As a result, residual metal from the substrates are often leached into the acidic samples when trying to recover Ba and should be removed subsequently by chemical purification techniques such as solvent extraction or ion exchange. Several methods have been investigated to separate Ba from other elements [3–6].
Working with near mono-isotopic solutions requires disciplined laboratory techniques to avoid unwanted contamination of the final product. The introduction of natural Ba to the enriched material is the most deleterious to the isotope ratios. Natural barium is the 14th most abundant element on earth with a crustal abundance of 390 ppm [7]. Ba can be found in metals, glassware, acids and other reagents, and in or on many laboratory items, so efforts should be made to work with pre-cleaned labware such as acid leached quartz and Teflon containers, and high purity (99.999 % or better) pre-cleaned collection foils. A clean room can also provide an environment that reduces the possibility of contaminating standards during the preparation stages.
Experimental
The stable isotope mass separator at INL, previously described in detail by Carney [2], was utilized for all of the isotope separation and collection described here. For the production of near mono-isotopic Ba, a starting material of approximately 1 g metallic, natural Ba is loaded into a hot cathode-type ion source; a modified model q-100 provided by Beam Imaging Solutions Inc. (previously Colutron Research Inc.). The Ba metal is heated in the source until it vaporizes and ionizes, forming a plasma. Ba ions are extracted from the plasma and accelerated at 35–40 kV. After the ions are accelerated, they travel through a quadrupole focusing lens, vertical steering plates, a 90° sector 1.5 m radius electromagnet, and finally a flight tube that is approximately the length of the magnet radius. At the end of the flight tube is a collection device (Fig. 1, left) that consists of V-shaped aluminum blocks that serve as Faraday cups. The blocks are designed to hold V-shaped collection foils (Fig. 1, right) onto which the separated isotopes are impinged, and are adjustable with regard to spacing in order to accommodate for elements of varying atomic masses. The apparatus is mounted to a plate containing slits approximately 32 mm high ×4 mm wide that are spaced for the collection of specific atomic mass unit ranges. For natural Ba that is processed using the mass separator (amu 130,132, 134–138), the distance of separation between each isotope with a 1 amu difference is approximately 12 mm. The slits for Ba collection were spaced approximately 12 mm apart and served as defining apertures for each collection block; this collimated the focused ions to ensure the best mono-isotopic collection onto each foil.
For initial collections, high purity Cu and Al foils (99.9999 %, Alfa Aesar) were used to collect isotopes because they did not contain detectable quantities of natural Ba (<200 ppt based on measurements of leach solutions). The foils were molded into a V-shape and mounted in the collection blocks. The separator was then operated for 10–20 h increments with beam currents of 134Ba around 100–200 nA. This level of throughput would typically yield a collection of 5–10 µg 134Ba and was considered sufficient to separate enough Ba without compromising mass resolution. After a typical isotope collection, the foils were carefully removed from the blocks and placed in polyethylene tubes that were filled with 3–6 M Optima® grade HNO3 of sufficient volume to cover the foils (typically 6–8 ml).This leached out the implanted isotopic Ba. After leaching the Ba for about 24 h, the acid was poured into separate containers and 0.25 ml aliquots were taken for ICP-MS analysis to determine purity and concentration of the recovered Ba isotopes. Leaching the Ba from the Al targets was requiredFootnote 1 and analyses to characterize leached Al and the mass of each Ba isotope that was deposited on each target was performed. The ratios of Ba isotopes in the solution were calculated by dividing the concentration of the major target isotope by the sum of the measured concentration of all seven Ba isotopes. The method limit of detectionFootnote 2 for each isotope was used in the calculation when the concentration of an isotope was below detectable levels. The isotopic abundance of each major isotope on each target varied between different periods of mass separator operation. For example, the 134Ba collected from one operation period may be enriched to greater than 98 %, while the 134Ba collected from another period may only be 90 % enriched. Mixing of these two target materials would decrease the enrichment of the more highly enriched isotope; as such, each target must be processed individually so not to compromise the isotopic abundance of the collected major isotope.
Aluminum concentrations in the solutions after the leaching process is performed can be as high as 1 mg Al g−1 acid after a typical 24 h Ba leach. This level can exceed the Ba concentration by a factor of 103 or higher. To separate the Ba from Al, experiments were conducted with column separations and solvent extractions to determine the best route for chemical purification.
Method for chemical purification of Ba solution on Eichrom Sr-spec cation exchange resin
Approximately 0.45 g of Sr-spec resin beads were loaded into a 18 cm polypropylene chromatography column (45–90 µm) filter (Evergreen Scientific) and washed with 2 ml 1 % (by vol.) Optima™ grade acetic acid to clean the resin. This was followed by a conditioning step with 2 ml 6 M HNO3. The cleaning and conditioning steps were performed in that sequence twice, ending with a final 6 M HNO3 contact to pre-equilibrate the resin for Ba solution loading. Aliquots of the last wash/conditioning steps were taken so they could be analyzed for total Ba and Al. The results of the analysis of a 2.37 g of a 6 M HNO3 solution containing leached quantities of 136Ba and Al is shown in Table 1. This solution was added to the resin and gently shaken for three minutes. The column was washed with 4 ml 6 M HNO3 to elute residual Al while Ba was retained on the resin. An aliquot of this wash was taken to be analyzed for Ba and Al. Ba was subsequently stripped from the column in three separate 2 ml batches of 0.01 M HNO3. Each batch was measured by ICP-MS for Ba and ICP-OES for Al to determine Ba recovery efficiency and overall separation of Ba from Al. A similar process was performed with the same 136Ba and Al solution diluted to 3 M HNO3, however, the column was pre-conditioned with 3 M HNO3 instead of 6 M HNO3. Each stage was replicated and analyses were performed to determine Ba recovery from the process as well as Ba separation from Al as a function of HNO3 concentration (3 vs. 6 M).
Method for chemical purification of Ba solution by solvent extraction
A 0.53 M crown ether solvent was prepared using 4,4′(5′)-di-t-butylcyclohexano-18-crown-6 dissolved in toluene. The solvent was washed two times with Optima™ grade 1 % (by vol.) acetic acid and pre-equilibrated with Optima™ grade 3 M HNO3. Each wash was agitated on a vortex shaker for 5 min. Aliquots of the acetic acid were analyzed for Ba and Al by ICP-MS and ICP-OES.
A 2 ml stock solution containing 137Ba enriched above 94 % and Al from the collection foil (Table 5) was agitated with the pre-cleaned solvent for 30 min on a vortex shaker. After the phases settled (separated), an aliquot was taken from the aqueous phase for Ba and Al analysis by ICP-MS and ICP-OES. Then 1 ml of the Ba-loaded solvent was stripped with 1 ml 0.01 M HNO3 and analyzed.
Method for isotope solution preparation
To test the feasibility of preparing spike material while maintaining a degree of enrichment, a trial run was conducted with 134Ba solutions that were 97 % enriched. The 99 % enriched solutions were to be saved until method validations were completed. Solvent extraction was the preferred route for chemical purification because it provided a high separation factor of Ba from Al.
Several 97 % enriched 134Ba solutions of HNO3 were combined and diluted to a concentration of 3 M with a total volume around 50 ml. These contained around 20 µg 134Ba. These solutions were agitated with the 0.53 M crown ether/toluene solvent on a shaker for up to 2 h. The organic solvent and aqueous phases were then separated by centrifugation for 5 min and pipetted into separate containers. The solvent was subsequently stripped (back-extracted) with approximately 13 ml 0.01 M HNO3 for 2 h to recover the Ba. The phases were centrifuged for 5 min, pipetted into separate containers, and the aqueous phase was brought to a final acid concentration of 1 M with a 134Ba concentration of about 300 ng 134Ba g−1acid. After the purification process, the solution was brought into a small modular class 1000 clean room (Fig. 3). The purified solution was dispensed into 5 Suprasil® quartz vials and 3 Savillex® Teflon vials (Fig. 2) using a gas pressurized extraction chromatography (GPEC) apparatus (Fig. 3). The GPEC system utilized a pre-measured length of Teflon tubing with a volume of 2 ml that was loaded with solution pumped from the stock container. After the tube was filled, the solution was dispensed slowly into a vial by gas pressure from an argon tank. This process was repeated until each vial was filled. The Teflon vials were capped and the quartz vials were flame sealed. The 134Ba enrichment of the stock solution was tracked throughout this process by analyzing aliquots taken before and after handling.
Results and discussion
As stated previously, stable isotopes of Ba were collected on Al foil substrates and leached into solution with HNO3. During this process, Al was partially dissolved but subsequently separated from the highly enriched Ba isotopes in order to produce chemically pure enriched Ba isotope solutions. This work showed significant retention of Al on the Sr specific resin. High concentrations of Al could detrimentally impact the retention of Ba due to competition for active sites on the resin. This degraded this extraction validated method, for example, to age date Cs sources using a micro-column based Cs–Ba separation. In addition, the effect of significant Al on the determination of Ba by thermal ionization mass spectrometry is unknown. Hence, the Al was removed from the Ba solutions.
Several extraction methods were tested using solutions that contained enriched 136Ba and 137Ba and residual Al target material. The Ba-enriched solutions were selected to assess if natural Ba was introduced during the Al removal process (measured by a change in the 138Ba isotopic abundance). The solid phase extraction was based on the method developed at INL to separate Ba from Cs and Sr from Zr. It has been shown to extract Ba efficiently and introduce minimal impurities. The solvent extraction method was ultimately selected due to simplicity, its ability to be performed rapidly, and its high selectivity of extraction of Ba over Al.
Liquid–solid (Sr-spec column) extractions
According to the EiChom literature, Sr-Resin is manufactured using a 1.0 M 4,4′(5′)-di-t-butylcyclohexano-18-crown-6 (crown ether) dissolved in 1-octanol. This solution is loaded onto an inert resin backing at a 40 % (w/w) loading.Footnote 3
A 136Ba solution was used to evaluate the resin based purification process. The result of the analysis of the 6 M HNO3 solution after rinsing the column material prior to the experiment is shown in the second row of Table 1. Part per million levels (17 µg g−1 solution) of Al contamination were measured, however no Ba contamination was detected. Similar concentrations were detected in the 6 M HNO3 solution used to condition the resin before use. The water blank (Optima™ grade) had detectable quantities of the major Ba isotopes (136Ba, 137Ba, and 138Ba). The measured isotope ratios of 136Ba–138Ba and 137Ba–138Ba in the water solution were 0.12 and 0.16 respectively. The expected ratios for naturally abundant Ba are 0.09 and 0.16 respectively. It appears that the ratios are close to natural abundance. Only 137Ba was detected in the acetic acid blank and it was at an insignificant level relative to the experiment concentrations; in fact no other Ba isotopes or Al were detected. The acetic acid wash of the resin prior to use showed the elution of detectable quantities of naturally abundant Ba and no detectable quantity of Al. The concentration of the Ba isotopes dissolved from the target and used in this separation is shown in the 8th row of Table 1. The abundance (enrichment) of the 136Ba isotope was 98.3 %. The concentration was 1530 ng 136Ba g−1 6 M HNO3 and 1210 µg Al g−16 M HNO3. The 136Ba analyses in the remaining 3 fractions of the 0.01 M HNO3 strip fractions account for 53 % of the initial 136Ba concentration. The Al mass was decreased to only 2.2 % of the original mass loaded through the column. Approximately 75 % of the Al was retained on the column. The concentration of Ba not accounted for could have remained on the column; inefficient stripping has been noticed during the Cs–Ba age dating procedure development and is typical when only dilute HNO3 is used. The measured isotopic enrichment of all the fractions throughout the experiment with the exception of the first 0.01 M HNO3 strip were maintained at greater than 98 %; the first strip fraction was reduced in purity to 92 %. A small but measurable additional quantity of 138Ba was detected and a smaller fraction of the 136Ba was present in this solution. The concentrations were converted to equivalent mass of Ba isotopes and Al in Table 2. This is required to determine the percent of 136Ba accounted for in each step of the loading or elution. Upon loading the Ba onto the column, the concentration of 136Ba in the 6 M HNO3 solution was measured to be 202 ng 136Ba g−1 6 M HNO3. The fraction of 136Ba accounted for in the load and rinse fractions is approximately 36 % of the initial 136Ba in solution. It is quite unusual for 36 % of the Ba in the 6 M HNO3 solution to not be retained on the resin; typically the column doesn’t exhibit this type of bleed-through, which may be due to inefficient packing, too high loading flow rates or competitive binding for sites on the resin from excessive Al. The experiment didn’t allow for these factors to be de-convoluted. More experimentation is required to determine if Al site competition is the reason for reduced Ba retention.
An identical experiment was repeated using 3 M HNO3 in place of 6 M HNO3 as the loading and rinsing solution. The results are provided in Tables 3 and 4. The 136Ba foil was leached and the 136Ba concentration was measured at 833 ng g−1 acid. The Al concentration was significantly lower than the previous run, being only 654 μg g−1 solution. The combination of reducing the acid concentration from 6 to 3 M and potentially the reduced Al concentration reduced the Ba rinsed through the column during the loading and rinse steps to only 6 %. The fraction of Ba recovered in the eluted 0.01 M HNO3 solutions was 70 % of the original solution. The enrichment level of 136Ba prior to and after the purification was 97 %. The mass of Al in the strip fractions was less than 2.5 % of the original quantity. Approximately 50 % of the Al was retained on the resin.
Liquid–liquid crown ether extractions
The concentration of Ba isotopes and Al in a 2 ml stock 3 M HNO3 solution containing greater than 94 % enriched 137Ba from a mass separator collection is provided in Table 5. This table also shows Ba and Al assay for each step of the extraction process.
Results shown in Table 6 indicate that very little contaminant Ba was in the solvent initially, and on the final wash, most or all of it was stripped. Approximately 70 % of the Ba from the stock solution was extracted into the solvent and was recovered in the final back-extraction (strip) step. Results suggest some Al may have been extracted into the solvent, but little or none was back-extracted with the Ba. Since only a fraction of the Ba-loaded solvent was back-extracted, and since the strip fraction was a smaller volume than the initial stock solution, it appears that more Ba was recovered than was initially introduced. Despite this observation, the concentration of Ba in the stock solution before and after extraction shows a Ba distribution ratio D = [org]/[aq] of 2.38. The separation factor of Ba and Al after the extraction step (D Ba/D Al) = (2.38/0.056) is approximately 42. Most of the Ba was recovered from the solvent in the back-extraction step, while the Al concentration that was back-extracted in the same fraction was below the detection limit of the instrument. The decontamination factor of Al from the Ba solution is considered highly acceptable in this case.
A previous scoping experiment using the same solvent but with a lower crown ether concentration (0.04 M) and an extraction contact time of 15 min yielded approximately 5 % Ba extraction. After the crown ether concentration was increased by over a factor of 13, and the contact time doubled, the Ba extraction was approximately 70 % (Table 6). Total Al recovery remained below detection limits in both cases.
In recent mass separator experiments, Ti and Au foils were used as collection substrates so that Ba could be selectively leached form the surface without dissolving the substrate material. Initial results showed that 134Ba can be collected on either substrate and isolated while maintaining enrichment levels above 99 %. Ti was preferred over Au because of lower cost and higher collection efficiency. The higher collection efficiency on the Ti foils has not been evaluated for this project, but it is likely related to differences in substrate density.Footnote 4 The solutions used to leach Ba from these foils do not require follow-up chemical purification because they did not contain detectable levels of the respective foil material. After the foils are leached, the solution can be adjusted to the desired acidity and Ba concentration and then used directly to appropriate among solution vials. In the former processes that require chemical purification, the back extraction (strip) fraction is used to fill the solution vials after it is adjusted to the proper acidity and Ba concentration.
134Ba material encapsulation
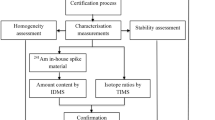
Previous work at INL [8] for the preparation of a certified reference materialFootnote 5 for determining the age since purification of a 137Cs sealed source using the ratio of 137Ba–137Cs showed that significant quantities of natural Ba contamination can be leached from commercially acquired quartz ampoules. For that reference material, INL prepared custom 5 ml volume quartz vials from Suprasil® quartz. Given this history, INL has prepared 100 5 ml Suprasil® quartz vials to encapsulate the 134Ba spike solution. Prior to solution encapsulation, the vials were washed in 3 M HNO3 for 24 h and then decanted. They were washed again for 24 h with weaker acid (approximately 1.1 M HNO3) which was then analyzed for Ba content. This step was performed until Ba levels were below the instrument detection limit. The vials were then filled using the GPEC system with Ba solution that was previously purified by the solvent extraction process to remove Al. The Ba concentration in the solution was adjusted to approximately 300 ng g−1 solution, and the acidity was adjusted to 1 M. The 97 % enriched Ba solution was analyzed before and after it was ran through the GPEC. This process performed in the clean room showed that the vials could be filled without introducing contamination. Samples encapsulated in both Suprasil® and Savillex® vials were sent to the National Institute of Standards and Technology (NIST) for analysis. Initial NIST analytical results indicate that natural Ba contamination was not introduced during encapsulation or opening of the vials. Analytical results from INL showed that 97 % enrichment was maintained after the solution was later opened after being encapsulated. Although Savillex® vials are essentially free from natural Ba contamination from the manufacturer; both types are suitable for spike storage if they are pre-cleaned.
Conclusions
INL produced significant quantities of high purity natural Ba isotopes and isolated them for future use as IDMS standards that are to be certified by NIST as Standard Reference Materials. The demonstration of methodologies for the collection, chemical purification, and encapsulation of Ba isotopes in a final solution form was considered a success.
Al and Cu foils work well as collection substrates because they are available in high purity forms (>99.999 %), but leaching these foils to recover Ba into HNO3 it necessitates chemical purification to remove dissolved foil material.
The evaluation of both solid–liquid and liquid–liquid extraction methods to remove Al from Ba-containing HNO3 solutions proved useful; both methods recovered approximately 70 % of the Ba. It was determined that the concentration of crown ether is important in obtaining significant extraction and recovery of Ba from the tested solution. As such it is believed that this can be improved with further optimization of either method by (1) increasing the ratio of crown ether by increasing the mass of resin or concentration dissolved in toluene, (2) increasing contact time for both systems, and (3) adding acetic acid into the strip solution.
To reduce the need for follow-up chemical purification of Ba solutions, pre-cleaned Ti and Au foils were used as collection substrates. The experiments demonstrated that metals with high resistance to dissolution by HNO3 could be used to collect Ba isotopes and did not contaminate the leaching solutions with either the foil material itself or natural Ba.
It was demonstrated by INL’s mass separator group that homogenous, enriched isotope solution sets for IDMS can be successfully prepared and made available to customers. Future plans include the separation and isolation of natural Zr and Sr isotopes to provide material for new standard reference materials (SRMs). There is continuing effort to increase separator throughput by improving ion source efficiency and by exploring various ion focusing and collection methods. The main goal of the effort is to increase beam current while maintaining high isotope selectivity.
Notes
The targets are not completely dissolved; attempts were made to adjust the acid type and concentration so to preferentially dissolve the Ba from the Al target using a leaching procedure. This still etched significant Al from the surface of the targets. Al foils are preferred over Cu foils for HNO3 dissolutions because they are not as easily dissolved during the Ba leaching process.
The instrument limit of quantification was 15 times the standard deviation of the blank divided by the slope of the calibrated instrument response for that specific isotope. The method limit of detection incorporated appropriate dilution factors that were run. The detection limit for each Ba isotope is approximately 3 ppt (ICP-MS). The detection limit for total Al is approximately 10 ppb (ICP-OES).
EiChrom Technical Data report: http://www.eichrom.com/eichrom/products/info/sr_resin.aspx.
There is a possible correlation between collection substrate density and ion collection efficiency. Substrates with higher density likely cause ions to back-scatter more readily, reducing the total number of ions collected.
This reference material is in its final stages of production/certification by INL, NBL and NIST.
References
Vogl J (2007) J Anal At Spectrom 22:475–492
Carney KP, Horkley JJ, McGrath CA, Edwards AJ, Davies JE, Knighton GC, Sommers JD, Giglio JJ (2013) J Radioanal Nucl Chem 296:383–387
Chabaux F, Ben Othman D, Birck JL (1994) Chem Geol 114:191–197
Horwitz EP, McAlister DR, Dietz ML (2006) Sep Sci Technol 41:2163–2182
Bagán H, Tarancón A, Rauret G, García JF (2011) Anal Chim Acta 686:50–56
Chiarizia R, Horwitz EP, Dietz ML, Cheng YD (1998) React Funct Polym 38:249–257
Greenwood NN, Earnshaw A (1984) Chemistry of the Elements. Pergamon Press plc., Elmsford, New York
Sommers J, Cummings D, Giglio J, Carney K (2009) J Radioanal Nucl Chem 282:591–595
Acknowledgments
The authors wish to thank Beam Imaging Solutions Inc., the Oak Ridge National Laboratory stable isotope mass separator group, the National Institute of Standards and Technology, and the U.S Department of Homeland Security for their continued support for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horkley, J.J., Carney, K.P., Gantz, E.M. et al. Production of highly-enriched 134Ba for a reference material for isotope dilution mass spectrometry measurements. J Radioanal Nucl Chem 305, 267–275 (2015). https://doi.org/10.1007/s10967-015-4047-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4047-z