Abstract
The paper deals with the seasonal variation of physico-chemical parameters in natural spring water sources collected from Pithoragarh city, Uttarakhand, India. A total of sixteen parameters were investigated for pre-monsoon and post-monsoon seasons during the study. Total dissolved solids have been found to range from 315 to 729 mg/L during pre-monsoon and 245 to 704 mg/L during post-monsoon. Total hardness and total alkalinity were observed to be high for two samples. Water quality index was evaluated to determine whether the use of water samples for drinking purpose is safe or not and its values range between 35.71 and 74.39 during pre-monsoon and 31.66 and 72.60 during post-monsoon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water has always been a subject of great concern due to our dependency on this inevitable source for the survival of life. In the last few years, a lot of studies have been undertaken for the assessment of the potability of water. Various chemicals present in water can cause adverse health effects and it depend upon the concentration of that particular chemical parameter. Like total dissolved solids (TDS) gives information about inorganic (calcium, magnesium, bicarbonate, chloride, and sulfate) and organic matters that are dissolved in water. Oxidation-reduction potential (ORP) is also an important parameter as it measures the ability of water to oxidize contaminants [1]. Human beings are exposed to natural radioactivity since the inception of this earth. Uranium (U) is one of the radioactive elements that is found naturally in the water and its presence in water above the safe limit becomes very dangerous to human health. According to a report, more than 33% of total groundwater sources are not safe for drinking purposes [2]. Various toxic elements are introduced to water day by day with progression of human civilization and they lead to water pollution. Different factors which are responsible are natural (through mineralization) as well as anthropogenic (through industrial and agricultural activities) resulting in serious threats to the health [3]. Various health risks like kidney damage [4], genotoxicity [5], breast cancer [6], and cardiovascular diseases can be caused due to the consumption of water containing uranium in long run [7, 8].
Various studies have been carried out worldwide to assess the water quality of springs. In 2019, a study was carried out in Turkey for the water quality assessment and sediment contamination assessment of Pazarsuyu stream [9]. Water quality assessment and microbial assessment was conducted in spring water samples of Beni–Mellal Atlas, Morocco [10].
Uranium is introduced to water through natural deposits, uranium industries, combustion of coal and use of phosphate fertilizers [11]. Initially, the World health organization (WHO) had proposed a guideline value of 15 µg/L but now it has been changed to 30 µg/L and according to Atomic Energy Regulatory Board (AERB), the permissible limit of uranium in drinking water is 60 µg/L. Most of the natural water sources contain a detectable amount of uranium [12]. In recent years, several studies have undertaken regarding the concentration of uranium in drinking water [1, 8, 12,13,14,15].
A study was carried out for the quality assessment of spring water samples for drinking purpose in the Himalaya of South Kashmir, India [16], Almora [17], Arunachal Pradesh [18], Kerala [19], Pauri [20]. The present study focuses on the seasonal variation of uranium concentration and other physico-chemical parameters in the spring water samples of Pithoragarh city. In hill areas, naturally flowing water is a prime source of drinking. Pithoragarh city also contains many springs which are serving people of the city from a long time. So, to confirm the potability of these springs, it becomes necessary to monitor these sources in a regular time interval. Uranium produce chemical toxicity as well as radiological toxicity. Keeping in view, the double toxicity of uranium and its health effects, the present analysis has been undertaken to assess uranium levels and other physicochemical parameters in the spring water sources of Pithogarh city. No similar type of study has been conducted in this area earlier. The occurrence of uranium and physico chemical parameters along with their correlation have been discussed in the present study.
Study area
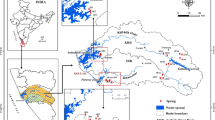
The study area covers nine sampling locations of Pithoragarh city. These locations have been widely used as sources of water by the surrounding residents. This area is represented by mountains bounded by main central thrust (MCT) in Lesser Himalayan region. The rocks of this zone cover crystalline thrust sheet in the form of large klippen masses mostly occupying the higher topographical levels of mountain ranges. Generally metamorphosed protrozoic rocks deposited by granites of variable ages along with metamorphosed to unmetamorphosed sedimentary rocks are extensively found in this range [21]. Particularly, the selected sampling sites are in Tejam group [22]. The rock type in the surroundings of all the sampling locations was found to be sedimentary, consisting Mandhali (Sor + Thalkedar) formations. The land in the surroundings of study area is used for agriculture all over the year. The map of the study area is represented in Fig. 1. Rock type with their corresponding formations and GPS coordinates of the sampling sites are represented in Table 1. Uranium dissolves immediately in oxygen- rich water which is responsible for its presence in groundwater, surface water and sea water. Maximum time dissolved uranium enters in water through weathering of igneous rocks [11].
Experimental
The samples were collected in a clean polypropylene bottle of 250 mL. The collection was done in pre-monsoon, April–May 2018 (PRM) and in post-monsoon seasons, November–December 2017 (POM). Samples were analyzed as soon as possible after the collection. Sixteen parameters were analyzed in the collected samples. GPS by Garmin Montana 680 has been used to identify the sampling locations. Various physico-chemical parameters like pH, total dissolved solids (TDS), electrical conductance (EC), temperature (T), oxidation-reduction potential (ORP), dissolved oxygen (DO), total hardness (TH), total alkalinity (TA), fluoride (F−), nitrate (NO3−), phosphate (PO4−3), sulphate (SO4−2), calcium (Ca+2), magnesium (Mg+2), Chloride (Cl−) and uranium (U) for collected samples were analyzed by following the standard protocols for estimation. For estimation of uranium, the samples were acidified by concentrated nitric acid up to pH 2 after collection.
Methods used for the analysis of physico-chemical parameters
pH, DO, TDS, EC, ORP, and T were analyzed immediately in the sampling site by using suitable probes by soil and water analysis kit (ITS-701) by ISO-TECH. The titration methods are standard and suitable methods to analyse TH, Ca–H, Cl− and TA parameters. For determining TH, ammonia buffer was added to the water sample followed by the addition of indicator Eriochrome Black T and ethylediamminetetra acetic acid (EDTA) as a titrant. TA was measured using phenolphthalein and methyl orange indicator. For the estimation of calcium- hardness (Ca–H), NaOH was added to the samples followed by the Murexide indicator and EDTA titrant. Mohr’s method was used for the analysis of Cl− concentration in the samples. Ca+2 and Mg+2 were calculated from Ca–H and Mg–H respectively by using the following equation:
[Ca+2] = Ca− hardness/2.5 [15]
[Mg+2] = Mg− hardness/4.11
Water quality index (WQI)
The water quality index (WQI) is an important parameter to determine the potability and sustainability of drinking water. For determining WQI, the standards prescribed by the Bureau of Indian Standards (BIS) and World Health Organization (WHO) are used. At first, all the parameters are assigned a weight or rank (wi) as per their importance in overall water quality for drinking purposes (Table 5) [15]. After that relative weight (Wi) is calculated by using the following equation
where,Wi= Relative weight, wi= weight/rank, n= number of parameters
The calculated relative weight is given in Table 5.
In the next step, quality assessment (qi) is done by using the following equation
where qi= quality rating Ci= concentration of parameter analyzed during the study Si= Standard value prescribed by BIS (2012)
Now, for calculating WQI, sub -index (SI) is determined for each parameter by using the following equation and then WQI is calculated by using the following equation
where SIi= sub- index of ith parameter
The classified range for water quality is given in Table 7 [15]. From this table we can easily analyze the potability of water.
Method used for the analysis of the uranium concentration
Laser fluorimeter model No. LF- 2A, with a wavelength of 400 nm was used for the analysis of uranium concentration, which is used to measure the U concentration in trace and ultratrace levels. The method for estimation of uranium concentration using laser fluorimeter in aquatic environment was produced by Laser Instruments Sections of Raja Ramanna Centre for Advanced Technology, Department of Atomic Energy, India. The source of laser fluorimeter was LED [11]. For analysis 5 mL of water sample was transferred to a dry and clean cuvette and 0.5mL of 5% sodium pyrophosphate (SPP) of pH 7 was added to the sample and measured. For calibration of instrument, standard uranium solution of known concentration was used. To avoid any error, micropipettes and analytical balance were used.
The formula used for the estimation of the concentration of U in water samples was
where D1 = Fluorescence due to sample only D2 = Fluorescence due to sample and U standard spiked V1= Volume of U standard added (mL)V2= Volume of sample taken (mL)C = Concentration of U− standard solution (µg L−1)
Result and discussion
Physico-chemical parameters
Statistical analysis of the physico-chemical parameters is represented in Table 2 with their corresponding standard values given by BIS and WHO [23, 24]. pH was reported to be slightly acidic to slightly alkaline. In both the seasons the value of pH is reported under the limit (6.5–7.5) prescribed by BIS. TDS ranged from 315 to 729 mg/L during PRM and 245–704 mg/L during POM. TDS value of two samples (Mahadev dhara (Market) and Marh–Kharayat) in PRM and three samples (Mahadev dhara (Market), Marh–Kharayat and Chaira) in POM were higher than the desirable limit of 500 mg/L as prescribed by BIS [23]. TDS and EC are two important parameters that tells about the concentration of soluble salts in water. Both the parameters have strong correlation between them. The range of EC during PRM is found to be 448–1103 µS/cm and 365–1052 µS/cm during POM. The study of regression analysis also reveals a strong association between TDS and EC (Fig. 2). The range of ORP lies between 165 and 261 mV during PRM and 178 and 252 mV during POM. The maximum value of fluoride concentration in water was 0.35 mg/L in PRM with an average of 0.16 mg/L, and 0.28 mg/L in POM with an average of 0.14 mg/L. Magnesium concentration was lower for Gurna (26.05mg/L) during PRM and also lower for Satsiling (23.31mg/L) during POM, than the prescribed value of 30 mg/L by BIS. Chloride also influences the TDS [25] and gives an idea about the salinity of water. A regression analysis was also done between TDS and Cl−, a good interrelation between both the parameters was observed with the R2 value equals to 0.58 (Fig. 3). In regression analysis TDS was taken as dependent variable while EC and Cl− were taken as independent variables. The average ionic concentration of parameters follows the order in Cl−>Mg+2>Ca+2>SO4−2> NO3−>PO4−3> F−during PRM and Cl−>Mg+2>Ca+2> NO3−>SO4−2> PO4−3> F−during POM. Uranium concentration during PRM was reported to be 0.1–0.70 µg/L with an average of 0.20 µg/L, and during POM it was found to be 0.1–0.64 µg/L with an average of 0.23 µg/L. A slight seasonal variation in physico-chemical parameters of drinking water samples has distinctly been observed in the study area may indicate that the vicinity of these natural water springs was not heavily altered on seasonal change.
Many studies in other parts of India have been undertaken for the assessment of uranium in drinking water which are mentioned below with their corresponding source of water and uranium concentration in Table 3. Sedimentary rocks are predominantly found in these study areas. Uranium is dependent on its oxidation state in which it prevails in the bed rock or surface rock and varies with the pH and ORP of water [26]. It is reported that if the ORP is high, there is high tendency of release and mobilization of U into deep aquifers [27, 28]. The average value of total alkalinity (TA) in PRM and POM is 272 and 301.33 mg/L respectively. The average value of pH is found below 8 in both seasons, i.e. there is no phenolphthalein alkalinity available or CO3−2 concentration is zero in the water samples but the bicarbonate ions are present in the form of alkalinity only. Following equations can be used for the estimation of CO3−2 and HCO3−.
PA<1/2 TA [18]
CO3−2= 2* PA
HCO3−−= TA− PA
Box- plots were plotted for pH, TDS, Cl−, SO4−2, TH and U which are represented in Fig. 4. The ends in the box -plot are called upper and lower quartiles. The midpoint is the median, which divides the box into two parts. The two lines above and below the box are called whisker and end at the highest and lowest value of data. In the box plot of TDS and Cl− the value of median represents the difference in seasonal variation of the parameters, while the median for SO4−2, TH and U represents that there is no noticeable change observed in both the seasons for these parameters.
A slight seasonal variation in physico-chemical parameters of drinking water samples has distinctly been observed in the study area may indicate that the vicinity of these natural water springs was not heavily altered on seasonal change.
Correlation analysis
Pearson correlation coefficient was used to determine the correlation between different physico-chemical parameters in both the seasons (Tables 4, 5). Uranium has good positive correlation with TDS (r= 0.77, p<0.05), EC (r=0.77, p< 0.05), and SO4−2 (r=0.73, p<0.05) during pre-monsoon while in post-monsoon significant positive correlation has been observed between U and TDS (r=0.74, p<0.05), EC (r=0.75, p<0.05), T (r=0.7, p<0.05), F− (r=0.77, p<0.05) and SO4−2 (r=0.87, p<0.01). Strong positive correlation of uranium with TDS and EC in both the seasons suggests that high TDS value in water samples implies the higher concentration of those ions which have a greater capacity to interact with uranium present in water [7]. TDS is showing a good positive correlation with most of the ions, TH, TA, and U. DO has significant negative correlation with TDS (r=− 0.9, p<0.01), EC (r=− 0.9, p<0.01), SO4−2 (r=− 0.8, p<0.05) and TH (r=− 0.7, p<0.05), TH has positive correlation with TA (r=0.75, p<0.05) and Mg (r=0.96, p<0.01) during pre-monsoon. DO has significant negative correlation with F− (r=− 0.69, p<0.05). Mg has strong positive correlation with TA (r=0.98, p<0.01) during post-monsoon. Especially positive correlation of uranium in both the seasons with TDS and EC specify the presence of uranium in its soluble higher oxidation states.
Assessment of Water quality index
Physico- chemical parameters were assigned a weight (wi) on the basis of their significance with respect to overall water quality for drinking purposes. The parameters with their relative weight and corresponding BIS standards are given in Table 6. A maximum weight of 5 has been assigned on the basis of the hazardous nature of the water quality parameter. U was assigned a weight of 5 due to its harmful effects [15]. Fluoride and nitrate are assigned a weight of 4, as they are major ion contributing to the quality of water (Table 7).
As mentioned in “Study area” section, by using equation 4, it is observed that the calculated WQI value lies between 35.71 and 74.39 during pre-monsoon and 31.66 and 72.60 during post-monsoon. The table representing the quality of water in collected water samples is given in Table 8. All the samples are either in the excellent category or in the good category. The map for water quality in the selected sampling sites is represented in Fig. 5. Water quality index pointed to use for drinking purpose as all samples were fall in excellent to good on the basis of chemical consideration, therefore, proper chlorination or boiling of water is recommended prior to use for safe drinking purpose.
Conclusion
-
The physico-chemical properties of water samples were analyzed and found that TDS of two samples was higher than the prescribed limit. Total hardness and total alkalinity were also found higher than their respective limits. There is no fluoride, nitrate pollution in the selected sampling sites.
-
Uranium concentration was analyzed for Pithoragarh city and found to be well within the prescribed value of WHO. Hence, the selected spring water samples are safe for drinking purposes with respect to uranium toxicity.
-
The correlation analysis shows a significant correlation of TDS with EC, SO4−2, TA and U during PRM. Similarly, U is also showing a strong correlation with TDS, EC and SO4−2.
-
Water quality index represents that two spring water samples were in excellent category and others were found to be in good category during PRM. Three samples were found to be in excellent category while others were in good category during POM.
-
The spring water in these selected areas is in good condition but requires continuous monitoring to avoid any water related issues in the future. Along with this, there is a great urge for educating the people of study areas through various awareness programs so that these natural spring water sources can be free from pollution and can be used for drinking for long.
Abbreviations
- PRM:
-
Pre-monsoon
- POM:
-
Post-monsoon
- U:
-
Uranium
- TDS:
-
Total dissolved solids
- ORP:
-
Oxidation-reduction potential
- EC:
-
Electrical conductance
- DO:
-
Dissolved oxygen
- T:
-
Temperature
- TA:
-
Total alkalinity
- TH:
-
Total hardness
References
Bajwa BS, Kumar S, Singh S, Sahoo SK, Tripathi RM (2017) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10:13–19
Chakraborti D, Das B, Murrill MT (2011) Examining India’s groundwater quality management. Environ Sci Technol 45:27–33
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Heal 8:311–329
Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Mäkeläinen I et al (2002) Renal effects of uranium in drinking water. Environ Health Perspect 110:337–342
Sahu M, Sar SK, Baghel T, Dewangan R (2020) Seasonal and geochemical variation of uranium and major ions in groundwater at Kanker district of Chhattisgarh, central India. Groundw Sustain Dev 10:1–9
Wagner SE, Burch JB, Bottai M, Puett R, Porter D, Bolick-Aldrich S et al (2011) Groundwater uranium and cancer incidence in South Carolina. Cancer Causes Control 22:41–50
Aswood MS, Jaafar MS, Salih N (2017) Estimation of annual effective dose due to natural radioactivity in ingestion of vegetables from Cameron Highlands, Malaysia. Environ Technol Innov 8:96–102
Sahu M, Sar SK, Baghel T, Dewangan R (2020) Seasonal and geochemical variation of uranium and major ions in groundwater at Kanker district of Chhattisgarh, central India. Groundw Sustain Dev 10:1–9
Ustaoğlu F, Tepe Y (2019) Water quality and sediment contamination assessment of Pazarsuyu Stream, Turkey using multivariate statistical methods and pollution indicators. Int Soil Water Conserv Res 7:47–56
Barakat A, Meddah R, Afdali M, Touhami F (2018) Physicochemical and microbial assessment of spring water quality for drinking supply in Piedmont of Béni-Mellal Atlas (Morocco). Phys Chem Earth 104:39–46
Sahoo SK, Mohapatra S, Chakrabarty A, Sumesh CG, Jha VN, Tripathi RM et al (2009) Distribution of uranium in drinking water and associated age-dependent radiation dose in India. Radiat Prot Dosimetry 136:108–113
Singh M (2019) Ground water year book, 2017–18 Uttarakhand. Central Groundwater Board, Dehradun, Uttarakhand
Sar SK, Diwan V, Biswas S, Singh S, Sahu M, Jindal MK, Arora A (2018) Study of uranium level in groundwater of Balod district of Chhattisgarh state, India and assessment of health risk. Hum Ecol Risk Assess 24:691–698
Sharma T, Sharma A, Kaur I, Mahajan RK, Litoria PK, Sahoo SK, Bajwa BS (2019) Uranium distribution in groundwater and assessment of age dependent radiation dose in Amritsar, Gurdaspur and Pathankot districts of Punjab, India. Chemosphere 219:607–616
Das A, Das SS, Chowdhury NR, Joardar M, Ghosh B, Roychowdhury T (2020) Quality and health risk evaluation for groundwater in Nadia district, West Bengal: an approach on its suitability for drinking and domestic purpose. Ground Sustain Dev 10:1–13
Pandey SS, Bholey S, Singh BM, Gautam YP, Balaram P (2020) Geochemical signatures and chemical toxicity assessment of groundwater uranium in the agricultural dominated tehsil after Indian summer monsoon. Res J Chem Environ 24:75–82
Lone SA, Bhat SU, Hamid A, Bhat FA, Kumar A (2021) Quality assessment of springs for drinking water in the Himalaya of South Kashmir, India. Environ Sci Pollut Res 28:2279–2300
Kumar K, Rawat DS, Joshi R (1997) Chemistry of springwater in Almora, Central Himalaya, India. Environ Geol 31:150–156
Bui Y, Lodhi MS, Regional NE, Pradesh A (2020) Assessment of spring water quality using water quality index method: study from upper Subansiri district, Arunachal Pradesh, India. Int J Environ Sci 9:898–908
Nair HC, Padmalal D, Joseph A (2015) Hydrochemical assessment of tropical springs: a case study from SW India. Environ Monit Assess 187:1–24
Chauhan JS, Badwal T, Badola N (2020) Assessment of potability of spring water and its health implication in a hilly village of Uttarakhand, India. Appl Water Sci 10:1–10
Valdiya KS (1980) In: Geology of Kumaun Lesser Himalaya. Wadia Institute of Himalayan Geology, Dehradun
Bureau of Indian Standards (BIS) (2012) Indian Standard Drinking Water- Specification (2nd Revision) IS 10500:2012, New Delhi
World Health Organization (WHO) (2011) Guidelines for drinking water quality (4th Edition), Geneva
Balakrishnan P, Saleem A, Mallikarjun ND (2011) Groundwater quality mapping using geographic information system (GIS): a case study of Gulbarga City, Karnataka, India African. J Environ Sci Technol 5:1069–1084
Waseem A, Ullah H, Rauf MK, Ahmad I (2015) Distribution of natural uranium in surface and groundwater resources: a Review. Crit Rev Environ Sci Technol 45:2391–2423
Yang Q, Smitherman P, Hess CT, Culbertson CW, Marvinney RG, Smith AE, Zheng Y (2014) Uranium and radon in private bedrock well water in maine: geospatial analysis at two scales. Environ Sci Technol 48:4298–4306
Ghosh S, Majumder S, Roychowdhury T (2019) Assessment of the effect of urban pollution on surface water- groundwater system of Adi Ganga, a historical outlet of river Ganga. Chemosphere 237:1–9
Singh B, Singh G, Sandhu AS, Singh S (1999) Urnaium estimation in water samples collected from Some Areas of Himachal Pradesh, India. Radiat Meas 31:683–685
Singh S, Malhotra R, Kumar J, Singh B, Singh L (2001) Uranium analysis of geological samples, water and plants from Kulu area, Himachal Pradesh, India. Radiat Meas 34:427–431
Ramola RC, Singh S, Virk HS (1989) Uranium and radon estimation in some water samples from himalayas. Nucl Tracks Radiat Meas 15:791–793
Balbudhe AY, Srivastava SK, Vishwaprasad K, Srivastava GK, Tripathi RM, Puranik VD (2012) Assessment of age-dependent uranium intake due to drinking water in Hyderabad, India. Radiat Prot Dosimetry 148:502–506
Singh S, Rani A, Mahajan RK, Walia TPS (2003) Analysis of uranium and its correlation with some physico-chemical properties of drinking water samples from Amritsar, Punjab. J Environ Monit 5:917–921
Babu MNS, Somashekar RK, Kumar SA, Shivanna K, Krishnamurthy V, Eappen KP (2008) Concentration of uranium levels in groundwater. Int J Environ Sci Technol 5:263–266
Mehra R, Singh S, Singh K (2007) Uranium studies in water samples belonging to Malwa region of Punjab, using track etching technique. Radiat Meas 42:441–445
Singh P, Rana NPS, Azam A, Naqvi AH, Srivastava DS (1996) Levels of uranium in waters from some Indian cities determined by fission track analysis. Radiat Meas 26:683–687
Bansal V, Tyagi RK, Prasad R (1988) Determination of uranium concentration in domestic water samples by fission track method. J Radioanal Nucl Chem Artic 125:439–443
Shashi PS, Bholey S, Singh BM, Gautam YP, Balaram P (2020) Geochemical signatures and chemical toxicity assessment of groundwater uranium in the agricultural dominated tehsil after indian summer monsoon. Res J Chem Environ 24:75–82
Patni K, Pande C, Pande AP, Tewari G, Joshi T (2020) Distribution of naturally occurring uranium and other heavy toxic elements in selected spring water samples of Pithoragarh District, Uttarakhand, India. SN Appl Sci. 2:1–9
Kumar A, Arora T, Singh P, Singh K, Singh D, Pathak PP, Ramola RC (2021) Quantification of radiological dose and chemical toxicity due to radon and uranium in drinking water in Bageshwar region of Indian Himalaya. Groundw Sustain Dev 12:100491
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest was reported by authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patni, K., Pande, C., Pande, A.P. et al. Seasonal variation of uranium and physico-chemical parameters in spring water sources of Pithoragarh city, Uttarakhand, India. J Radioanal Nucl Chem 329, 647–660 (2021). https://doi.org/10.1007/s10967-021-07823-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07823-8