Abstract
(HDEHP + Hexa)/SiO2-P, a silica-based adsorbent, was prepared and used to separate Y(III) from a mixed solution of Sr(II) and Y(III). The adsorption behavior of the adsorbent was investigated using batch tests. The adsorbent exhibited high adsorption performance for Y(III) in low nitric acid concentrations and weak adsorption performance for Y(III) at higher concentrations. In contrast, Sr(II) was not adsorbed in either acid concentration range. The same tendency was observed under hydrochloric acid conditions. Y(III) separation from a mixed solution of Sr(II) and Y(III) was verified by a column test. Overall, the (HDEHP + Hexa)/SiO2-P adsorbent can separate Y(III) from Sr(II).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-level liquid waste (HLLW) generated by spent nuclear fuel reprocessing includes large quantities of Sr and Y isotopes (~1.1 kg/1 tHU, 45 GWd/t) [1, 2]. 90Sr and 90Y are the main isotopes of Sr and Y that generate considerable heat in HLLW. 90Sr decays to 90Y by emitting a β-ray with a half-life of approximately 28 years.
90Y is a radioisotope used as a radiopharmaceutical in nuclear medicine for various therapeutic applications, for example therapeutic agent for Malignant lymphoma. As 90Y is present in mixed solutions of 90Sr and 90Y, it is vital to separate 90Y from the solution to be used as a radiopharmaceutical [3, 4]. 90Y is the major daughter nuclide in the 90Sr decay system and emits 2.28 MeV β-rays due to radioactive decay with a half-life of approximately 64 h to become a stable daughter nuclide, 90Zr. This is represented by the following reaction:
Therefore, the selective separation of 90Sr and 90Y from HLLW is essential from the point of view of nuclear waste management.
Yttrium is a rare earth metal used in various applications such as radiation therapy and materials for industrial products. Nevertheless, to use it, it is essential to separate yttrium from mixtures with other metals. In recent years, various methods for separating yttrium from metals mixture have been studied. The separation of yttrium by a precipitation method was reported. In this study, a method of separating yttrium from a CRT (cathode ray tube) was reported to reduce waste and recover and reuse valuable metals from the viewpoint of environmental protection. In this method, the CRT was dissolved in sulfuric acid, and the yttrium in the resulting solution was recovered by precipitation with C2H4O2 (Valentina et al.) [5]. A solvent extraction method and an ion exchange method to extract yttrium from a mixed solution of rare earth elements have been evaluated. An extraction system using sec-octylphenoxy acetic acid (CA12) and bis (2,4,4-trimethylpentyl) phosphinic acid (Cyanex272) achieved the high-purity separation of yttrium from rare earth ores using a solvent extraction method (Yanliang et al.) [6]. In addition, yttrium was separated selectively from a mixed solution of rare earth elements using an ion-exchange method on a C18 column with tetra-n-butylammonium hydroxide (TBAOH) as the ion interaction reagent and nitrilotriacetic acid (NTA) as the complexing agent (Rajmunds et al.) [7]. The separation of yttrium using ionic liquids has also been reported; bifunctional ionic liquids [CA12] [methyltrioctyl ammonium (N1888)] could separate yttrium from rare earth concentrates (Yanliang et al.) [8]. Research on extraction chromatography using an adsorbent with an organic extractant immobilized on a solid phase was also conducted. The daughter nuclide, 90Y, was separated from seawater using diglycolamide (DGA) resin and measured to monitor the 90Sr released into the ocean due to the incident at the Fukushima Daiichi Nuclear Power Plant (Tazoe et al.) [9].
A microporous silica/polymer composite carrier (SiO2-P) has been used to fix the organic extractant. SiO2-P is an inorganic/organic hybrid material prepared by impregnating a macroporous SiO2 substrate with a copolymer. SiO2-P is a promising carrier for extractants due to its strong acidity, mechanical strength, radiation resistance, and easy separation of solids and liquids. Research on separation using adsorbents with various extractants impregnated into SiO2-P has also been conducted. Y can be separated from a mixed solution of Sr(II) and Y(III) using a N,N,N′,N′-tetra-n-octyl-diglycolamide (TODGA) impregnated adsorbent (Xu et al.), a 4'4'(5')-di(tert-butylcyclohexano)-18-crown-6 (DtBuCH18C6) impregnated adsorbent (Kim et al.) or an octyl(phenyl)-N,N-diisobutylcarbamoyl methylphosphine oxide (CMPO) impregnated adsorbent (Kawamura et al.) [10,11,12].
In this study, bis-(2-ethylhexyl) phosphoric acid (HDEHP) was used as an extractant; Fig. 1 shows its molecular structure. HDEHP is a trivalent cation extractor. Because Y(III) exists as a trivalent cation in solution, Y(III) might be separated from a mixed solution of Sr(II) and Y(III) using HDEHP. The above research is summarized in Table 1.
The silica-based HDEHP adsorbent was prepared by impregnating HDEHP and 1-hexanol as a molecule modifier into SiO2-P. The effects of the acid concentration, contact time, temperature, and metal ion concentration for the adsorbent were investigated to understand Y(III) adsorption and separation. Y(III) was separated from a mixed solution of Sr(II) and Y(III) with HDEHP adsorbent using a column test.
Experimental
Materials
HDEHP was obtained from Tokyo Chemical Industry Co., LTD. Sr(NO3)2 (98%) and SrCl2 (95%) were obtained from Wako Pure Chemical Industries, Ltd. Y(NO3)3·6H2O (99.99%) and YCl3·6H2O (99.99%) were supplied by Kanto Chemical Co.
Preparation of adsorbent
In this study, the adsorbent was prepared by impregnating the HDEHP extractant into SiO2-P and 1-hexanol (Hexa) as a molecule modifier into SiO2-P. The SiO2-P particles were washed three times with approximately 300 cm3 of methanol and vacuum dried for 1 day. The extractant HDEHP was dissolved in approximately 200 cm3 dichloromethane, used as a diluent, with a molecule modifier 1-hexanol. SiO2-P was mixed with dichloromethane. After the mixture was stirred for approximately 1.5 h, the dichloromethane was removed using a rotary evaporator. The residue was vacuum dried at 313 K for one day and the adsorbent (HDEHP+Hexa)/SiO2-P was obtained [12].
Batch method
The Sr(II) and Y(III) adsorption behaviors onto (HDEHP+Hexa)/SiO2-P were examined using a batch method. Approximately 0.2 g of the dry adsorbent was divided into 13.5 cm3 glass vials, and 4 cm3 of the liquid solution was added to the glass vials. The glass vials were shaken at 160 rpm for 10 min to 5 h in a thermostatic shaking bath. The solution was either HNO3 or HCl containing different concentrations of Sr (II) and Y (III), each having different acid concentrations. [12]
The adsorbents and the solution were separated by filtration after shaking. The concentrations of the metal ions in the solution were measured by inductively coupled plasma-atomic emission spectrometry (ICP-AES, Shimadzu ICPE-9000). The distribution coefficients (Kd, cm3/g) and uptake ratios (R, %) of the Sr(II) and Y(III) ions were calculated as follows:
where C0 and Ce are the concentrations of metal ion before and after adsorption in the solution in ppm, respectively; m is the dry adsorbent weight in g, and V is the solution volume in cm3.
Column method
Approximately 3 g of (HDEHP+Hexa)/SiO2-P adsorbent and water were placed in a glass beaker, and the adsorbent was degassed by suction. Subsequently, the adsorbent was packed into a glass column (8 mm inner diameter × 100 mm length) and prepared.
The column test was conducted under two conditions: nitric acid and hydrochloric acid. Several solutions were passed through the column using a metering pump. The temperature of the column was maintained at 298 K using a thermostatic water jacket, and the flow rate during the column test was approximately 0.5 cm3/min.
Under the nitric acid condition, 0.5 M HNO3 solutions containing 10 mM Sr(II) and Y(III) were fed into the column as the feed solutions. A 0.5 M HNO3 solution containing no metal ions was fed continuously into the column as a washing solution. 3 M HNO3 was also fed into the column after 0.5 M HNO3 to elute the metal ions adsorbed on the adsorbent. As the solutions were fed to the column, the solution out from the column was collected in 5 ml tubes using a fraction collector. ICP-AES measured the Sr(II) and Y(III) concentrations in each tube. Under the hydrochloric acid conditions, the experiment was carried out in the same procedure [12].
Results and discussion
Effect of the acid concentration
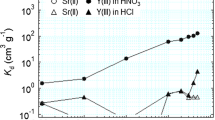
A batch test was conducted to understand the effects of the acid concentration on the adsorption behavior [10,11,12,13,14,15]. Figure 2 shows the effects of the acid concentration on the (HDEHP+Hexa)/SiO2-P adsorbent. Under nitric acid conditions, the (HDEHP+Hexa)/SiO2-P adsorbent showed high adsorption affinity for Y(III) in the range of low acid concentrations (0.001–0.1 M). At higher acid concentrations, the adsorption affinity decreased with increasing acid concentration, and the uptake ratio was a few percent from 3 to 5 M HNO3. On the other hand, Sr(II) was barely adsorbed onto the adsorbent at any acid concentration.
Under the HCl condition, the tendency of adsorption was similar to the case of the HNO3 condition. Sr(II) was barely adsorbed onto the adsorbent at any acid concentration. Y(III) was well adsorbed onto the (HDEHP+Hexa)/SiO2-P adsorbent at low acid concentrations, and the ratio decreased at higher acid concentrations. On the other hand, the difference was large at a 1 M acid concentration. The uptake ratio under the HNO3 condition was more than 80%, whereas it was less than 50% under the HCl condition. Therefore, (HDEHP+Hexa)/SiO2-P adsorbent showed good selectivity for the adsorption of Y(III) at low acid concentration.
Peppard proposed the extraction of trivalent lanthanides by HDEHP and reported that the metal (M3+) is coordinated to three HDEHP dimers (HDEHP)2. [16] The mechanism can be represented by eq. (4):
As the H+ concentration in the solution increases, the chemical equilibrium shifts to the left, and Y(H(DEHP)2)3 decreases, according to Eqs. (4). This suggests that the adsorption weakens as the acid concentration increases, regardless of the type of acid.
These results suggest that it is possible to separate Y(III) and Sr(II) with a low acid concentration (<0.5 M) and elute Y(III) with a high acid concentration (>3 M).
Effect of contact time
A batch test was conducted to understand the effects of the contact time on the adsorption behavior. The kinetic data of the adsorption was fitted to a pseudo-second-order equation:
where Qt and Qe are the amounts of Y(III) adsorbed at time t and equilibrium (mg/g), respectively; k2 is the rate constant of the pseudo-second-order adsorption (g/mg h). These models are commonly used to describe adsorption behavior. In this model, the adsorption process seemed to be chemical adsorption, and the adsorption capacity is proportional to the number of active sites. [17,18,19,20]
Figure 3 shows plots of t/Qt versus time. Figure 4 shows plots of Qt versus time fitted to the pseudo-second-order kinetic model. In Fig.3, plots of t/Qt versus time showed a straight line, and the correlation coefficient (R2) was approximately 1.00. This indicates that the adsorption process was fitted well to the pseudo-second-order model.
Qe and k were calculated from the slope of the straight line and are summarized in Table 2. The Qe value was determined to be 0.23 mmol/g and 0.24 mmol/g under the HNO3 and HCl conditions, respectively. Although the experimental value was lower than the calculated Qe, a value close to the calculated value can be obtained by continuing until the reaction reaches equilibrium.
Effects of temperature
A batch test was conducted at 288 K, 298 K, and 308 K under the HNO3 and HCl conditions to understand the effects of temperature on the adsorption behavior. The thermodynamic properties of adsorption of Y(III) to (HDEHP+Hexa)/SiO2-P were investigated by calculating some thermodynamic parameters using from these results Van't Hoff's equation [21,22,23].
The Van’t Hoff equation is expressed as
where ΔG0 is the change in Gibbs free energy (kJ/mol), ΔH0 is the change in standard enthalpy (kJ/mol) and ΔS0 is the change in standard entropy (J/K·mol). T is the temperature (K), and R is the gas constant (8.314 J/mol·K); Kd is the same as Eq. (2).
Figure 5 shows plots of ln(Kd) versus 1/T. Both relationships were linear, and the correlation coefficient (R2) was approximately one. The ln(Kd) value increased as 1/T was increased, and the slope was larger under the HNO3 condition than under the HCl condition. ΔH0 and ΔS0 were determined from the slope and intercept of the plots of ln(Kd) vs. 1/T. The values of ΔH0 and ΔS0 were negative under both solution conditions. The adsorption processes of Y(III) on (HDEHP+Hexa)/SiO2-P adsorbent were exothermic. ΔG0 was calculated for each temperature and summarized in Table 3. The values of ΔG0 for each temperature were negative and decreased with increasing temperature. Hence, the adsorption reaction progresses spontaneously, and it readily adsorbs at high temperatures.
Adsorption isotherm
The adsorption mechanism was assessed by conducting a batch test in HNO3 and HCl solutions with various metal concentrations and compared using three adsorption models: Langmuir equation, Freundlich equation, and Dubinin–Raddushkevitch (D–R) equation. [24,25,26] The adsorption isotherm shows the relationship between the adsorbate concentration at equilibrium and the amount of adsorbate on the adsorbent.
The Langmuir equation represents adsorption, assuming that the adsorption sites are distributed uniformly and are monolayered. The Dubinin–Raddushkevitch equation assumes that the adsorption sites are non-uniform. The Freundlich equation is an empirical formula for adsorption equilibrium; it is usually applied to multi-layer and non-uniform surface adsorption.
The Langmuir equation, Freundlich equation and Dubinin–Raddushkevitch equation are given by the following:
where Ceq (mol/L) and Qeq (mol/g) are the equilibrium concentration of Y(III) in the aqueous and solid phases, respectively. qm (mol/g) is the maximum capacity of Y(III) taken up. KL (L/mol), Kf (mol/g), and Kad (mol2/kJ2) are the Langmuir constant, Freundlich constant, and Dubinin–Radushkevich constant, respectively. n is an exponential constant related to the adsorption strength of the Freundlich model. R is the gas constant (8.314 J/mol K) and T is the temperature (K).
Table 4 summarizes the values of each constant, the maximum capacity, and the correlation coefficients for Y(III) ions. Figure 6 shows a non-linear relationship in each model. A comparison of the parameters closely resembled the Langmuir model under both conditions, suggesting that both adsorption processes appear to be single-layer adsorption.
In the Langmuir model, the maximum adsorption capacity of Y(III) on the (HDEHP+Hexa)/SiO2-P adsorbent was 0.26 mmol/g under HNO3 conditions and 0.31 mmol/g under HCl conditions.
Column separation
A column test was conducted to determine if Y could be separated and recovered from a mixed solution of Sr(II) and Y(III). Figure 7 presents the extraction chromatography results of separating Y(III) from a mixed solution of Sr(II) and Y(III) using (HDEHP+Hexa)/SiO2-P adsorbent in HNO3 or HCl solutions. In a HNO3 solution, Sr(II) was not adsorbed and immediately flowed out of the column. On the other hand, Y(III) was retained. Subsequently, when H2O was supplied to the column, the Y(III) adsorbed on the adsorbent was eluted from the column. The behavior was similar under the HCl solution conditions to that under the HNO3 conditions.
The elution rate of Y(III) from the column was different. A clear elution peak of Y(III) was observed when the HNO3 solution was used, but the elution of Y(III) was continuous when the HCl solution was used.
The chemical yield of Y(III) from the start of elution and end of elution was calculated to be approximately 90 wt% under the HNO3 solution. The column test showed that it was possible to separate and recover Y(III) from a mixed solution of Sr(II) and Y(III) using the (HDEHP+Hexa)/SiO2-P adsorbent.
Table 5 compares the present result with the results of separation using the TODGA, DtBuCH18C6, and CMPO adsorbents reported elsewhere. Although the recovery rate of the HDEHP adsorbent was slightly lower than that of the other adsorbents, a high recovery rate of 90% was still achieved. The HDEHP adsorbent showed considerably higher Kd and Qe values than the other adsorbents in nitric acid. This is because the complex of yttrium with HDEHP has a stronger bond than with the other extractants. These results indicate that the amount of yttrium adsorbed onto the HDEHP adsorbent is more than the other adsorbents when the amount of adsorbent is the same.
In addition, the k2 value was the second-fastest after the 1-dodecanol-added CMPO adsorbent, confirming that the adsorption rate was sufficiently high. Therefore, there is no need to slow down the flow rate of the feed solution to the column or lengthen the column.
This suggests that the amount of adsorbent required may be less than the other adsorbents. Moreover, HDEHP adsorbents appear to be effective in making the separation system compact.
Conclusions
The Sr(II) and Y(III) adsorption behaviors on the adsorbent under various conditions (different concentrations of acids (HNO3 or HCl), contact times, temperatures, and concentration of metal ions) were investigated using a batch method.
The adsorption of Y(III) on the adsorbent showed high adsorptivity at low acid (HNO3 and HCl) concentrations (0.001–0.5 M), but the adsorption weakened as the acid concentration was increased. On the other hand, Sr(II) was barely adsorbed. These results suggest that Y(III) can be separated from a mixed solution of Sr(II) and Y(III) using this adsorbent. Regarding the effect of the contact time, the adsorption of Y(III) on the adsorbent was well fitted to a pseudo-second-order model (R2 ≈ 1.0). In addition, it took several hours to reach equilibrium, but the adsorption reaction proceeded immediately after contact, and approximately half of the Y(III) in equilibrium was adsorbed within 30 min to 1 h. Regarding the effect of temperature, the results of the test showed that these adsorption processes proceeded spontaneously. The adsorption mechanism was analyzed using the Langmuir, Freundlich, and Dubinin–Raddushkevitch isotherms. The Langmuir model provided the best correlation with Y(III) adsorption on the adsorbent, indicating that the adsorption mechanism is single-layer adsorption.
The separation and recovery behavior of Y(III) from a mixed solution of Sr(II) and Y(III) using the adsorbent was tested using the column method. In the HNO3 solution, separation and recovery of Y(III) were achieved, and the recovery rate was approximately 90%. On the other hand, Y(III) was eluted gradually under the HCl conditions, which was inefficient. Therefore, HNO3 appears to be better than HCl.
The separation and recovery of Y(III) from a mixed solution of Sr(II) and Y(III) was achieved, and it was confirmed that this (HDEHP+Hexa)/SiO2-P adsorbent has sufficient capacity to separate Y(III) from a mixed solution of Sr(II) and Y(III).
References
Zhamg A, Kuraoka E, Kumagai M (2007) Development of the chromatographic partitioning of cesium and strontium utilizing two macroporous silica-based calix[4]arene-crown and amide impregnated polymeric composites: PREC partitioning process. J Chromatogr A 1157:85–95
Wu Y, Kim SY, Tozawa D, Ito T, Tada T, Hitomi K, Kuraoka E, Yamazaki H, Ishii K (2012) Equilibrium and kinetic studies of selective adsorption and separation for strontium using DtBuCH18C6 loaded resin. J Nucl Sci Technol 49:320–327
Chakravarty R, Pandey U, Manolkar RB, Dash A, Venkatesh M, Pillai MRA (2008) Development of an electrochemical 90Sr-90Y generator for separation of 90Y suitable for targeted therapy. Nucl Med Biol 35:245–253
Lee JS, Park UJ, Son KJ, Han HS (2009) One column operation for 90Sr/90Y separation by using a functionalized-silica. Appl Radiat Isot 67:1332–1335
Innocenzi V, Michelis ID, Ferella F, Beolchini F, Kopacek B, Vegliò F (2013) Recovery of yttrium from fluorescent powder of cathode ray tube, CRT: Zn removal by sulphide precipitation. Waste Manag 33:2364–2371
Wang Y, Liao W, Li D (2011) A solvent extraction process with mixture of CA12 and Cyanex272 for the preparation of high purity yttrium oxide from rare earth ores. Sep Purif Technol 82:197–201
Tian F, Sun X, Liu X, Zhang H, Liu J, Guo H, Zhang Y, Meng C (2020) Effective adsorptive denitrogenation from model fuels over yttrium ion-exchanged Y zeolite. Chin J Chem Eng 28:414–419
Wanga Y, Huanga C, Li F, Donga Y, Zhaoa Z, Sun X (2016) The development of sustainable yttrium separation process from rare earth enrichments using bifunctional ionic liquid. Sep Purif Technol 162:106–113
Tazoe H, Obata H, Yamagata T, Karube Z, Nagai H, Yamada M (2016) Determination of strontium-90 from direct separation of yttrium-90 by solid phase extraction using DGA Resin for seawater monitoring. Talanta 152:219–227
Xu Y, Kim SY, Ito T, Nakazawa K, Funaki Y, Tada T, Hitomi K, Ishii K (2012) Adsorption and separation behavior of yttrium and strontium in nitric acid solution by extraction chromatography using a macroporous silica-based adsorbent. J Chromatogr A 1263:28–33
Kim SY, Kawamura T, Ito T (2019) Adsorption of Sr(II) and Y(III) by extraction chromatography using DtBuCH18C6-impregnated adsorbent, Global 2019, Seattle, WA, 22–27 Sept 2019
Kawamura T, Ito T, Kim SY (2019) Adsorption and separation behavior of strontium and yttrium using a silica-based CMPO adsorbent. J Radioanal Nucl Chem 320:9–14
Kudo T, Ito T, Kim SY (2017) Adsorption behavior of Sr(II) from high-level liquid waste using crown ether with ionic liquid impregnated silica adsorbent. Energy Procedia 131:189–194
Zhang A, Xiao C, Liu Y, Hu Q, Chen C, Kuraoka E (2009) Preparation of macroporous silica-based crown ether materials for strontium separation. J Porous Mater 17:153–161
Dutta S, Mohapatra PK, Raut DR, Manchanda VK (2011) Chromatographic separation of carrier free 90Y from 90Sr using a diglycolamide based resin for possible pharmaceutical applications. J Chromatogr A 1218:6483–6488
Tkac P, Vandegrift GF, Lumetta GJ, Gelis AV (2012) Study of the interaction between HDEHP and CMPO and its effect on the extraction of selected lanthanides. Ind Eng Chem Res 51:10433–10444
Ho YS, Mckay G (1999) Pseudo-second order model for sorption process. Process Biochem 34:451–465
Lin J, Wang L (2009) Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front Environ Sci Eng 3(3):320–324
Naushad M, Al Othman ZA, Awual MR, Alam MM, Eldesoky GE (2015) Adsorption kinetics, isotherms, and thermodynamic studies for the adsorption of Pb2+ and Hg2+ metal ions from aqueous medium using Ti(IV) iodovanadate cation exchanger. Ionics 21:2237–2245
Wu H, Kim SY, Miwa M, Matsuyama S (2021) Synergistic adsorption behavior of a silica-based adsorbent toward palladium, molybdenum, and zirconium from simulated high-level liquid waste. J Hazard Mater 411:125136
Lima EC, Gomes AA, Tran HN (2020) Comparison of the nonlinear and linear forms of the van’t Hoff equation for calculation of adsorption thermodynamic parameters (∆S° and ∆H°). J Mol Liq 311:113315
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J Hazard Mater 190:916–921
Ueberbacher R, Rodler A, Hahn R, Jungbauer A (2010) Hydrophobic interaction chromatography of proteins: thermodynamic analysis of conformational changes. J Chromatogr A 1217:184–219
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J Appl Chem 3(1):28–45
Zhang A, Hu Q (2010) Adsorption of cesium and some typical coexistent elements onto a modified macroporous silica-based supramolecular recognition material. Chem Eng J 159:58–66
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawamura, T., Wu, H. & Kim, SY. Adsorption and separation behavior of strontium and yttrium using a silica-based bis(2-ethylhexyl) hydrogen phosphate adsorbent. J Radioanal Nucl Chem 329, 1001–1009 (2021). https://doi.org/10.1007/s10967-021-07806-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07806-9