Abstract
An three-dimensional (3D) porous structure graphene oxide nanoribbons (GONRs) aerogel has been prepared via hydrothermal method to overcome the challenges of solid–liquid separation for powdered carbon-based nanomaterials. GONRs aerogel showed low density, good mechanical strength and easy separation from water. Uranium(VI) and thorium(IV) adsorption by GONRs aerogel was investigated by batch experiments, demonstrating their strongly pH-dependent, spontaneous and endothermic adsorption processes. GONRs aerogel exhibited the maximum U(VI)- and Th(IV)-uptake capacity (430.6 and 380.4 mg g−1, respectively) due to its large specific area (597.4 m2 g−1) and abundant oxygen-containing groups. This work suggests that GONRs aerogel has great potential for treatment of uranium and thorium-containing effluents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium and thorium are important nuclear fuel and often used to generate electricity. However, uranium and thorium are dangerous in terms of radioactivity and chemical toxicity, which may cause the negative effect on living organism upon entry into living body [1]. The nuclear fuel cycles including the front-end and back-end are main sources of uranium and thorium-containing wastewater [2]. Therefore, it is indispensable to treat uranium and thorium from the contaminated sites for environmental conservation, human well-being and nuclear energy sustainability. Currently, there are several conventional methods for treating uranium and thorium in wastewater, such as ion exchange, solvent extraction, electrochemical, membrane separation and adsorption [3]. Among of above methods, adsorption has been widely used for the treatment of uranium and thorium in wastewater owing to its economy and universality [4, 5]. Up to now, various adsorbents for uranium and thorium adsorption have been reported, such as clay minerals [6], silica materials [7], polymers [8], carbon family materials [9, 10]and biological adsorbents [11].
Carbon family materials, especially carbon-based nanomaterials such as carbon nanotubes (CNTs) and graphene, are being employed for uranium and thorium uptake because of their good radiation resistance, excellent mechanical and thermal stability. In recent years, graphene nanoribbons (GNRs), a novel quasi-one-dimensional carbon-based nanomaterials, have attracted much attention. GNRs are strips of graphene with nanometer widths and possess many excellent properties that are different from other carbon-based nanomaterials [12]. Compared to the common graphene, GNRs have excellent flexibility and adjustability, are more suitable materials for structural purposes, which have been extensively employed in numerous research fields like sensing, catalysis, electrochemistry and adsorption [13, 14]. Our group has long been interested in developing GNRs-based adsorbents and reported on the first application of graphene oxide nanoribbons (GONRs) for wastewater treatment [15, 16]. Especially, several GONRs and their composites were prepared in our laboratory for the capture of Th(IV) [17,18,19]. However, due to the difficulty of solid–liquid separation after adsorption and the risk of possible secondary pollution to the environment, these powdered carbon-based nanomaterials are still limited in practical applications. In order to compensate for the above drawbacks, magnetic separation technology is widely used to realize rapid and effective separation for magnetic particles. Recently, interests in magnetic carbon-based nanomaterials have exploded and their significant potentials have been demonstrated for the treatment of radioactive wastewater [20, 21]. Unfortunately, the most significant shortcoming of magnetic carbon-based nanomaterials is instability in air, acids or bases, thus degrading their performance [22].Therefore, it is of crucial importance to explore new type of carbon-based nanomaterials composites with easy separation of solid–liquid and strong acid–alkali resistance.
Three-dimensional (3D) carbon-based nanomaterials have porous network structure, large specific surface area and good mechanical stability, which makes it promising as one of the most popular adsorption materials in the future [23,24,25]. Carbon nanomaterials aerogels, especially graphene aerogels, have been demonstrated numerous applications in radioactive wastewater fields [26, 27].For example, He et al. [28]. synthesized 3D porous network graphene oxide (GO) hydrogels with good mechanical stability for U(VI) adsorption, and found that the maximum adsorption capacity at pH 4.0 could reach 134.23 mg·g−1. Zhao et al. [26]. prepared an ultra-light graphene aerogel (GA) by a simple hydrothermal method, and the GA had a high adsorption capacity for U(VI) (238.67 mg·g−1at pH 4.0). Moreover, in order to further improve selectivity, self-assembled CA-PO4 was fabricated for U(VI) adsorption and revealed high selectivity of 66.8% at pH 5.5. Up to now, most carbon nanomaterials aerogels as adsorbents were prepared using CNTs and graphene as matrix materials, but little research focused on assembling GNRs into aerogels for radionuclides adsorption. In fact, 3D porous GNRs aerogel has been widely applied in the field of material science, environment and energy due to their superior physical and chemical characteristics [29, 30].
Herein, we have synthesized 3D porous GONRs aerogel derived from unzipping of CNTs by a simple hydrothermal method for U(VI) and Th(IV) adsorption. Various techniques were employed to characterize the physical and chemical properties of GONRs aerogel. Adsorption isotherms, kinetics and thermodynamics of U(VI) and Th(IV) on GONRs aerogel were comprehensively evaluated by batch experiments.
Experimental
Materials
The raw material of CNTs was purchased from China XFNano Material Technology Company. All the analytical-grade reagents used in this experiment, such as hydrogen peroxide (H2O2), nitric acid (HNO3), potassium permanganate (KMnO4), sodium hydroxide (NaOH), sulfuric acid (H2SO4) and ethanol, were procured from Sinopharm Chemical Regent Co., Ltd. (China).
Synthesis of GONRs aerogel
The synthetic procedure of GONRs was referenced by previous work [16]. The fabrication process of GONRs aerogel by hydrothermal method has been put into effect in our laboratory as described in the following steps (Scheme 1). Typically, 0.25 g of the above GONRs was weighed and dispersed through sonication into 15 mL of deionized water. Then the obtained homogeneous solution was transferred into the sealed teflon-lined stainless steel vessel (20 mL) and heated at 180 °C for 24 h. After the reaction kettle was cooled to room temperature, the black and cylindrical solid was taken out and cleaned repeatedly with ethanol and deionized water to prepare GONRs hydrogel. Subsequently, the as-prepared hydrogel was frozen at − 20 °C and completely lyophilized at − 55 °C for 24 h to obtain a black and cylindrical GONRs aerogel (GONRs-A). The resultant aerogel with a density of 6.2 mg·cm−3 could stand stably on top of a bristlegrass without breaking it, demonstrating its ultralight property [31, 32].The cylindrical GONRs aerogel could support several coins and bear more than 1000 times of itself own weight without deforming, further proving its good mechanical strength.
Characterization
X-ray power diffractions (XRD) patterns were measured on a D8 advance diffractometer with Cu Kα radiation. A Hitachi S-4800 scanning electron microscope (SEM) was applied to obtain the morphological images. Thermal behavior was conducted on a Q50 model thermogravimetric analyzer (TGA) by heating each sample from 20 to 800 °C under nitrogen atmosphere. PerkinElmer 100 spectrometer was used to obtain The Brunauer–Emmett–Teller (BET) surface area was determined by a Micromeritics Tristar instrument. Fourier transformed infrared (FT-IR) spectra were acquired using PerkinElmer 100 spectrometer. X-ray photoelectron spectroscopy (XPS, Thermofisher Escalab 250Xi) was used to identify the chemical composition of samples. The Zeta potential values were determined by a Particle Metrix flowing current potential analyzer (Microtrac, America).
Batch adsorption experiments
Typically, GONRs aerogel (10 mg) was weighted into 50 mL of U(VI) or Th(IV) solution with a given concentration and pH. Negligible HNO3 and NaOH were dropwise added to adjust the pH of solution. The mixture was kept agitating for indicated periods in a thermostatic oscillator at a certain temperature. Thereafter, the supernate was directly taken out and the concentrations of U(VI) and Th(IV) were detected by Optima 8000 model ICP-OES. Detailed description of the adsorption capacity qe (mg g−1) can be found in our previous work [19].
Results and discussion
Characterizations
Figure 1a–c exhibited morphological structures of CNTs, GONRs and GONRs aerogel by SEM. The original CNTs with hollow structure were curved and entangled (Fig. 1a), whose diameters and lengths were 10–50 nm and about a few micrometer, respectively. GONRs preserved the one-dimensional nanostructure of CNTs with widths of 30–100 nm and length extending to a few micrometer range (Fig. 1b). GONRs aerogel was assembled into 3D porous and interconnected network by randomly interleaved GONRs based on partially overlapping π-π interactions (Fig. 1c), forming micro- and nanoscale combined hierarchical structure [29].
To further investigate the structural evolution, XRD of GONRs and GONRs aerogel were performed with the results shown in Fig. 2a. GONRs exhibited a strong diffraction peak at 2θ = 10.0°, specifying the (001) refection plane and d-spacing of 8.6 Å, of typical graphene oxide [33]. For GONRs aerogel, the (001) peak disappeared and another dominate diffraction peak at 26.0° (d-spacing of 3.46 Å) could be observed. The interplanar spacing significantly decreased, indicating the formation of the network of GONRs aerogel by π-π interaction [34].
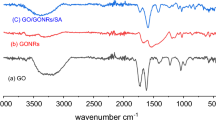
The changes of functional groups in GONRs and GONRs aerogel were analyzed by FT-IR and the results are shown in Fig. 2b. Both GONRs and GONRs aerogel exhibited eight characteristic bands: the band at 3440 cm−1 belonged to the -OH vibrations; the C-H bonds were visible at 2920 cm−1 and 2850 cm−1; the band at 1580 cm−1 may be related to graphite structure of CNTs; the band at 1384 cm−1 was associated with the disordered structure of carbon nanomaterials [35]; three peaks at 1050 cm−1, 1220 cm−1 and 1714 cm−1 were corresponded to the C–O–C, C–O and C=O stretching vibrations [36], respectively, reflecting the abundance of oxygen-containing groups on two adsorbents. Compared to GONRs, the peak intensities of GONRs aerogel at 1714 cm−1 and 1050 cm−1 improved tremendously, which implies more oxygen-containing groups formed in GONRs aerogel.
Figure 2c showed the TGA curves of GONRs and GONRs aerogel. The TGA curves of two samples consisted of three main stages. In the first stage, a slow mass loss below 150 °C could be ascribed to water dehydration. The second stage was from 150 to 500 °C, the prominent mass losses were present for the oxygen-containing groups decomposition [37], at which GONRs and GONRs aerogel presented the mass losses of approximately 17.6% and 28.8%, respectively. This indicated that GONRs aerogel possessed more abundant oxygen-containing groups than GONRs, which was consistent to FT-IR results. Up to 500 °C, the weight loss tended to become relatively slow, which was attributed to the burning of carbon atoms. The TGA curve of GONRs aerogel revealed a significant amount of the oxygen-containing groups and good thermal stability.
Figure 2d presented N2 adsorption–desorption isotherms of two samples. The calculated BET specific surface areas of the as-synthesized GONRs and GONRs aerogel were 83.8 and 597.4 m2 g−1, respectively. After hydrothermal treatment, the BET specific surface area increased significantly due to the formation of 3D porous network [26, 28]. Based on BJH method, the average pore diameter of GONRs aerogel was calculated to be 34.4 nm, which confirmed the mesoporous structure, thus facilitating the adsorption process.
As illustrated in Fig. 3a, two peaks centering at 284.6 eV (C 1 s) and 532.5 eV (O 1 s) were found in the XPS survey spectra of two adsorbents [37], confirming the presence of oxygen-containing groups. The high-resolution C 1 s spectra in Fig. 3b showed five peaks at 284.1, 285.1, 286.4 eV, 287.2 and 288.8 eV, respectively, which could be assigned to the sp2-hybridized graphite-like carbon atoms (C=C), the sp3-hybridized carbon atoms (C–C), hydroxyl groups (–C–OH), carbonyl groups (C=O) and carboxyl groups (–COO–). Moreover, It can be seen that GONRs aerogel had more oxygen-containing functional groups than GONRs aerogel. Together with previous FT-IR results, XPS analyses indicated that oxygen-containing functional groups were dramatically introduced during hydrothermal treatment.
Effect of initial solution pH
The influences of solution pH on U(VI) and Th(IV) removal by aerogel were shown in Fig. 4a. In this study, the pH was kept lower than 4.5 to prevent the hydrolysis of uranyl and thorium ions [38].The adsorption capacity of U(VI) and Th(IV) by GONRs aerogel increased with the increasement of pH. At lower pH value, the active sites were taken up by more H+ or H3O+, consequently declining the adsorption capacity of UO22+ or Th4+ by GONRs aerogel due to the competition effect. As pH increased, U(VI) and Th(IV) gradually transformed into hydrated species, thereby favoring the adsorption of U(VI) and Th(IV) by GONRs aerogel. Moreover, the surface charges of aerogel can affect the adsorption of U(VI) and Th(IV), and the point of zero charge of GONRs aerogel was tested to be 3.6 (Fig. 4b). GONRs aerogel had a negative zeta potential value when pH > 3.6, thus promoting the binding of uranyl and thorium ions with negative charges [38].
Adsorption kinetics
Figure 5a presented the relationship of U(VI) and Th(IV) adsorption capacities changing with contact time. Clearly, the adsorption rates of U(VI) and Th(IV) by GONRs aerogel were quite rapid in the first 10 min, and then gradually slowed down before reaching equilibrium within 60 min. The kinetic data were correlated with three kinetic model [2, 27], i.e. the pseudo-first order model, pseudo-second order model and the intra-particle diffusion model, to describe the kinetic characteristics of U(VI) and Th(IV) on GONRs aerogel. The corresponding fitting parameters were given in Table 1. The adsorption of GONRs aerogel for two actinides was portrayed better by pseudo-second-order with a higher correlation coefficient (Fig. 5a). The computed adsorption capacities (167.3 and 67.7 mg g−1 for U(VI) and Th(IV)) from pseudo-second-order model were also much closer to the experimental values (53.5 and 64.5 mg g−1 for U(VI) and Th(IV)). These results suggest that the rate-controlling step of U(VI) and Th(IV) on GONRs aerogel may be chemisorption [27].
a Effect of contact time on U(VI) and Th(IV) adsorption by GONRs aerogel and adsorption kinetic plots of pseudo-first-order and pseudo-second-order models (pH = 4.5 and C0 = 60 mg·L−1 for U(VI), pH = 3.0 and C0 = 30 mg·L−1 for Th(IV), w = 10 mg, t = 240 min, T = 298 K, and V = 50 mL). b the fitting plots of intra-particle diffusion model
Moreover, the simulation of intra-particle diffusion (Fig. 5b) showed three divided linear segments and the intra-particle diffusion rate constants accorded with the order of kint1 > kint2 > kint3. A rapid uptake occurred at the first stage because of the binding of U(VI) and Th(IV) by the outer chemical sites, then the uptake rate became slower at the second stage with the entry of ions into GONRs aerogel, and finally the adsorption reached saturation at the third stage. Hence, it could be concluded that not only chemical reaction but also mass transport jointly contributed to the adsorption of U(VI) and Th(IV) by GONRs aerogel.
Adsorption isotherms
Figure 6a exhibited the effect of changing metal ions concentrations on U(VI) and Th(IV) adsorption by GONRs aerogel. Obviously, as the initial concentrations of U(VI) and Th(IV) increased, the adsorption capacity of U(VI) and Th(IV) by GONRs aerogel progressively increased, finally the saturation was attained under higher concentration. To further clarify the adsorption behavior, the adsorption isotherms were investigated based on Langmuir, Freundlich and Dubinin-Radushkevich (D-R) models [3]. The fitting curves of these models were shown in Fig. 6 and the calculated data of different isotherm models were listed in Table 2. It was noted that the isotherms were more suitable for Langmuir isotherm model with a higher correlation coefficient, demonstrating that a monolayer coverage was formed on solid–liquid interface [38]. Also, The qm of U(VI) and Th(IV) by GONRs aerogel from Langmuir model were computed to be 430.6 and 380.4 mg g−1, respectively. Moreover, the experimental results were also in good agreement with the D-R isotherm model (Fig. 6b). The value of mean free energy (EDR) can be related to the aosorption mechanism [39]. The calculated EDR values of U(VI) and Th(IV) adsorption on GONRs aerogel were 9.068 and 8.562 kJ mol−1, respectively, demonstrating a chemisorption mechanism.
a Effect of the initial uranium and thorium concentration on adsorption by GONRs aerogel, and adsorption isothermal plots of Langmuir and Freundlich models (pH = 4.5 for U(VI) and 3.0 for Th(IV), w = 10 mg, t = 240 min, T = 298 K and V = 50 mL). b Best-fit plots of D-R model for U(VI) and Th(IV) adsorption on GONRs aerogel (pH = 4.5 for U(VI) and 3.0 for Th(IV), w = 10 mg, t = 240 min, T = 298 K, and V = 50 mL)
Adsorption thermodynamics
The relationship of U(VI) and Th(IV) adsorption capacities changing with temperature on was presented in Fig. 7a. The increases of adsorption capacities for U(VI) and Th(IV) were observed as temperature raised, suggesting that high temperature promoted the adsorption processes. To learn more about the adsorption thermodynamics, three crucial thermodynamics parameters containing ΔH, ΔS and ΔG, were analyzed by Eqs. 1–2.
The values of ΔH and ΔS were obtained from the slope and intercept of the linear relationship of lnKd versus T−1 (Fig. 7b). The corresponding values of ΔG were presented in Table 3. The calculated parameters for ΔH and ΔS were positive, indicative of the endothermic adsorption processes and the increasing randomness with the rise of temperature. Moreover, ΔG was calculated to be negative, revealing that U(VI) and Th(IV) adsorption by GONRs aerogel were thermodynamically feasible and spontaneous.
Comparison of adsorption capacity
Undoubtedly, adsorption capacity is a crucial parameter for adsorbents. Therefore, the maximum adsorption capacities of U(VI) and Th(IV) on various carbon nanomaterials-based adsorbents were compared to further evaluate the adsorption performance of GONRs aerogel, as shown in Table 4. Obviously, the U(VI) and Th(IV) adsorption capacity of GONRs aerogel outperformed all other previously reported pristine CNTs and graphene-based adsorbents. However, comparing the adsorption capacities of other functionalized carbon nanomaterials, the unfunctionalized GONRs aerogel exhibited the lower adsorption capacity. Even so, GONRs aerogel still has some noteworthy advantages over the other carbon nanomaterials-based adsorbents, such as 3D porous architecture, large specific surface area, good durability and easy solid–liquid separation, which makes it very promising for U(VI) and Th(IV) adsorption.
Conclusions
In conclusion, 3D porous network structure GONRs aerogel was successfully assembled for uranium(VI) and thorium(IV) adsorption. The obtained aerogel with low density, good mechanical strength and large specific surface area exhibited good adsorption performance, and the maximum U(VI)- and Th(IV)- capacity of GONRs aerogel for U(VI) and Th(IV) was 430.6 and 380.4 mg g−1, respectively, much more than most of the reported carbon nanomaterials. Chemisorption played a crucial role in U(VI) and Th(IV) adsorption on abundant oxygen-containing groups GONRs aerogel. The characteristics of easy separation from water and outstanding adsorption efficiency make GONRs aerogel attractive for U(VI) and Th(IV) capture.
References
Gui W, Zhang H, Liu Q, Zhu X, Yang Y (2014) Recovery of Th(IV) from acid leaching solutions of bastnaesite at low concentrations. Hydrometallurgy 147–148:157–163
Anirudhan TS, Suchithra PS, Senan P, Tharun AR (2012) Kinetic and equilibrium profiles of adsorptive recovery of thorium(IV) from aqueous solutions using poly(methacrylic acid) grafted cellulose/bentonite superabsorbent composite. Ind Eng Chem Res 51:4825–4836
Rao TP, Metilda P, Gladis JM (2006) Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination-an overview. Talanta 68:1047–1064
Hao J-H, Wang Z-J, Wang Y-F, Yin Y-H, Jiang R, Jin Q-H (2015) Adsorption of alkali and alkaline-earth metal atoms on the reconstructed graphene-like BN single sheet. Solid State Sci 50:69–73
Li J, Wang X, Zhao G, Chen C, Chai Z, Alsaedi A, Hayat T, Wang X (2018) Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem Soc Rev 47:2322–2356
Sharma P, Tomar R (2008) Synthesis and application of an analogue of mesolite for the removal of uranium (VI), thorium (IV), and europium (III) from aqueous waste. Microporous Mesoporous Mater 116:641–652
Humelnicu D, Blegescu C, Ganju D (2014) Removal of uranium (VI) and thorium (IV) ions from aqueous solutions by functionalized silica: kinetic and thermodynamic studies. J Radioanal Nucl Ch 299:1183–1190
Liu L, Liu S, Peng H, Yang Z, Tang A (2020) Surface charge of mesoporous calcium silicate and its adsorption characteristics for heavy metal ions. Solid State Sci 99:106072
Li F, Yang Z, Weng H, Chen G, Lin M, Zhao C (2018) High efficient separation of U(VI) and Th(IV) from rare earth elements in strong acidic solution by selective sorption on phenanthroline diamide functionalized graphene oxide. Chem Eng J 332:340–350
Jiang D, Liu L, Pan N, Yang F, Li S, Wang R, Wyman IW, Jin Y, Xia C (2015) The separation of Th(IV)/U(VI) via selective complexation with graphene oxide. Chem Eng J 271:147–154
Mahanty B, Mohapatra PK (2020) Highly efficient separation of thorium from uranium in nitric acid feeds by solid phase extraction using Aliquat 336. Sep Purif Technol 237:116318
Higginbotham AL, Kosynkin DV, Sinitskii A, Sun Z, Tour JM (2010) Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano 4:2059–2069
Long D, Li W, Qiao W, Miyawaki J, Yoon SH, Mochida I, Ling L (2011) Partially unzipped carbon nanotubes as a superior catalyst support for PEM fuel cells. Chem Commun 47:9429–9431
Xie L, Wang H, Jin C, Wang X, Jiao L, Suenaga K, Dai H (2011) Graphene nanoribbons from unzipped carbon nanotubes: atomic structures, Raman spectroscopy, and electrical properties. J Am Chem Soc 133:10394–10397
Wang Y, Wang Z, Gu Z, Yang J, Liao J, Yang Y, Liu N, Tang J (2015) Uranium(VI) sorption on graphene oxide nanoribbons derived from unzipping of multiwalled carbon nanotubes. J Radioanal Nucl Ch 304:1329–1337
Wang Y, Wang Z, Ang R, Yang J, Liu N, Liao J, Yang Y, Tang J (2015) Synthesis of amidoximated graphene oxide nanoribbons from unzipping of multiwalled carbon nanotubes for selective separation of uranium(VI). RSC Adv 5:89309–89318
Xiu T, Liu Z, Wang Y, Wu P, Du Y, Cai Z (2019) Thorium adsorption on graphene oxide nanoribbons/manganese dioxide composite material. J Radioanal Nucl Ch 319:1059–1067
Wu P, Wang Y, Li Y, Hu X, Xiu T, Yuan D, Liu Y, Wu Z, Liu Z (2019) Adsorption of Th(IV) from aqueous solution by the graphene oxide nanoribbons/chitosan composite material. J Radioanal Nucl Ch 322:553–559
Wu P, Wang Y, Hu X, Yuan D, Liu Y, Liu Z (2019) Synthesis of magnetic graphene oxide nanoribbons composite for the removal of Th(IV) from aqueous solutions. J Radioanal Nucl Ch 319:1111–1118
Zong P, Wang S, Zhao Y, Wang H, Pan H, He C (2013) Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions. Chem Eng J 220:45–52
El-Maghrabi HH, Abdelmaged SM, Nada AA, Zahran F, El-Wahab SA, Yahea D, Hussein GM, Atrees MS (2017) Magnetic graphene based nanocomposite for uranium scavenging. J Hazard Mater 322:370–379
Xiao J, Song W, Hu R, Chen L, Tian X (2019) One-step arc-produced amino-functionalized graphite-encapsulated magnetic nanoparticles for the efficient removal of radionuclides. ACS Appl Nano Mater 2:385–394
Bryning MB, Milkie DE, Islam MF, Hough LA, Kikkawa JM, Yodh AG (2007) Carbon Nanotube Aerogels. Adv Mater 19:661–664
Hu H, Zhao Z, Wan W, Gogotsi Y, Qiu J (2013) Ultralight and highly compressible graphene aerogels. Adv Mater 25:2219–2223
Sui Z, Meng Q, Zhang X, Ma R, Cao B (2012) Green synthesis of carbon nanotube-graphene hybrid aerogels and their use as versatile agents for water purification. J Mater Chem 22:8767–8771
Zhao D, Wang Y, Zhao S, Wakeel M, Wang Z, Shaikh RS, Hayat T, Chen C (2019) A simple method for preparing ultra-light graphene aerogel for rapid removal of U(VI) from aqueous solution. Environ Pollut 251:547–554
Zhang Z, Dong Z, Wang X, Dai Y, Cao X, Wang Y, Hua R, Feng H, Chen J, Liu Y (2019) Synthesis of ultralight phosphorylated carbon aerogel for efficient removal of U (VI): batch and fixed-bed column studies. Chem Eng J 370:1376–1387
He Y-R, Li S-C, Li X-L, Yang Y, Tang A-M, Du L, Tan Z-Y, Zhang D, Chen H-B (2018) Graphene (rGO) hydrogel: a promising material for facile removal of uranium from aqueous solution. Chem Eng J 338:333–340
Wang Q, Wang X, Chai Z, Hu W (2013) Low-temperature plasma synthesis of carbon nanotubes and graphene based materials and their fuel cell applications. Chem Soc Rev 42:8821–8834
Zhao F, Wang L, Zhao Y, Qu L, Dai L (2017) Graphene oxide nanoribbon assembly toward moisture-powered information storage. Adv Mater 29:1604972
Shan C, Zhao W, Lu XL, O’Brien DJ, Li Y, Cao Z, Elias AL, Cruz-Silva R, Terrones M, Wei B (2013) Three-dimensional nitrogen-doped multiwall carbon nanotube sponges with tunable properties. Nano Lett 13:5514–5520
Wu X-L, Wen T, Guo H-L, Yang S, Wang X, Xu A-W (2013) Biomass-derived sponge-like carbonaceous hydrogels and aerogels for supercapacitors. ACS Nano 7:3589–3597
Kosynkin DV, Higginbotham AL, Sinitskii A, Lomeda JR, Dimiev A, Price BK, Tour JM (2009) Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458:872–876
Zhu X, Yang C, Wu P, Ma Z, Shang Y, Bai G, Liu X, Chang G, Li N, Dai J, Wang X, Zhang H (2020) Precise control of versatile microstructure and properties of graphene aerogel: Via freezing manipulation. Nanoscale 12:4882–4894
Shao D, Jiang Z, Wang X, Li J, Meng Y (2009) Plasma induced grafting carboxymethyl cellulose on multiwalled carbon nanotubes for the removal of UO22+ from aqueous solution. J Phys Chem B 113:860–864
Li Z, Chen F, Yuan L, Liu Y, Zhao Y, Chai Z, Shi W (2012) Uranium(VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chem Eng J 210:539–546
Liao Y, Wang M, Chen D (2019) Electrosorption of uranium (VI) by highly porous phosphate-functionalized graphene hydrogel. Appl Surf Sci 484:83–96
Ding H, Zhang X, Yang H, Luo X, Lin X (2019) Highly efficient extraction of thorium from aqueous solution by fungal mycelium-based microspheres fabricated via immobilization. Chem Eng J 368:37–50
Chandrasekar A, Suresh A, Joshi M, Sundararajan M, Ghanty TK, Sivaraman N (2019) Highly selective separations of U(VI) from a Th(IV) matrix by branched butyl phosphates: Insights from solvent extraction, chromatography and quantum chemical calculations. Sep Purif Technol 210:182–194
Fasfous II, Dawoud JN (2012) Uranium (VI) sorption by multiwalled carbon nanotubes from aqueous solution. Appl Surf Sci 259:433–440
Chen J-H, Lu D-Q, Chen B, Yang P-K (2012) Removal of U(VI) from aqueous solutions by using MWCNTs and chitosan modified MWCNTs. J Radioanal Nucl Ch 295:2233–2241
Schierz A, Zanker H (2009) Aqueous suspensions of carbon nanotubes: surface oxidation, colloidal stability and uranium sorption. Environ Pollut 157:1088–1094
Wang Y, Gu Z, Yang J, Liao J, Yang Y, Liu N, Tang J (2014) Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium(VI). Appl Surf Sci 320:10–20
Zhao G, Wen T, Yang X, Yang S, Liao J, Hu J, Shao D, Wang X (2012) Preconcentration of U(VI) ions on few-layered graphene oxide nanosheets from aqueous solutions. Dalton Trans 41:6182–6188
Shao D, Hou G, Li J, Wen T, Ren X, Wang X (2014) PANI/GO as a super adsorbent for the selective adsorption of uranium(VI). Chem Eng J 255:604–612
Chen C, Li X, Zhao D, Tan X, Wang X (2007) Adsorption kinetic, thermodynamic and desorption studies of Th(IV) on oxidized multi-wall carbon nanotubes. Colloids Surf A: Physicochem Eng Asp 302:449–454
Deb AKS, Mohanty BN, Ilaiyaraja P, Sivasubramanian K, Venkatraman B (2012) Adsorptive removal of thorium from aqueous solution using diglycolamide functionalized multi-walled carbon nanotubes. J Radioanal Nucl Ch 295:1161–1169
Pan N, Deng J, Guan D, Jin Y, Xia C (2013) Adsorption characteristics of Th(IV) ions on reduced graphene oxide from aqueous solutions. Appl Surf Sci 287:478–483
Pan N, Guan D, He T, Wang R, Wyman I, Jin Y, Xia C (2013) Removal of Th4+ ions from aqueous solutions by graphene oxide. J Radioanal Nucl Ch 298:1999–2008
Bai Z-Q, Li Z-J, Wang C-Z, Yuan L-Y, Liu Z-R, Zhang J, Zheng L-R, Zhao Y-L, Chai Z-F, Shi W-Q (2014) Interactions between Th(IV) and graphene oxide: experimental and density functional theoretical investigations. RSC Adv 4:3340–3347
Xu H, Li G, Li J, Chen C, Ren X (2016) Interaction of Th(IV) with graphene oxides: Batch experiments, XPS investigation, and modeling. J Mol Liq 213:58–68
Acknowledgements
We appreciate the financial support from Jiangxi Key Laboratory for Mass Spectrometry and Instrumentation (East China University of Technology) (JXMS202015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., He, H., Liu, Z. et al. A facile method for preparing three-dimensional graphene nanoribbons aerogel for uranium(VI) and thorium(IV) adsorption. J Radioanal Nucl Chem 328, 289–298 (2021). https://doi.org/10.1007/s10967-021-07619-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07619-w