Abstract
This paper reports the extraction and separation of no-carrier-added (NCA) 195,195m,197mHg radioisotopes produced in 20 MeV 1H irradiated Au target. The extraction studies were carried out from HNO3 media by solid–liquid extraction using TK200 and DGA-N resins based on trioctylphosphine oxide (TOPO) and N,N,N,N-tetra-n-octyldiglycolamide (TODGA) respectively. TK200 resin was found to be the superior extractant for separation of NCA Hg radionuclides from 3 M HNO3 with a separation factor of 3.2 × 105.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The radioisotope of Hg, 197mHg (T½ = 23.8 h, IT = 91%, Eγ = 133.9 keV) is used in single photon emission computed tomography (SPECT) for lung tumour imaging since 1950s [1]. It is also a potential new therapeutic radionuclide as proposed by Neves et al. [2] along with 19 other suitable radioisotopes due to its auger and conversion emission electrons, thereby, listed as a theranostic radionuclide candidate.
The common production routes of 195,195m,197mHg radioisotopes are deuteron [3, 4] or proton induced reactions on Au target [1, 5,6,7]. These studies used stacked foil technique to measure the excitation function and production cross sections of 197Au(d,xn) or 197Au(p,xn) reactions. Tárkányi et al. [3] measured the cross-sections of deuteron induced nuclear reactions on gold target upto 40 MeV projectile energy and later they extended the same measurement upto 50 MeV projectile energy [4]. Alpha or 3He induced reactions on platinum target has been explored for production of mercury radioisotopes [8, 9]. Sudar and Qaim [9] measured the excitation function of natPt(α,xn)192,193m,193g,195m,195g,197m,197g,199mHg reactions upto 37 MeV projectile energy while Hermanne et al. [8] studied the same reaction in the projectile energy range 17–26 MeV. Heavy ions like 7Li bombardment on Au target [10, 11] or platinum target [11] also produced 197mHg indirectly by the decay of 197Tl. Available cross section data for production of 197m,gHg radionuclides and other Hg radionuclides have been tabulated in Table 1. Ghosh et al. [12] mentioned that although several ways of Hg production are possible, use of 197Au(d,x) or 197Au(p,xn) reactions involve the production of least amount of contaminants from the radioisotopes of other elements, which is also evident from Table 1.
There are few reports available on radiochemical studies involving the separation of the no-carrier-added (NCA) Hg radioisotopes either from bulk Au target or from NCA Au radionuclides. Lahiri et al. [13] produced neutron deficient 192,193Hg and 192,193Au radionuclides via heavy ion induced reaction 181Ta(16O,xn)192,193Tl (\(\epsilon\))192,193Hg (\(\epsilon\))192,193Au. Conventional liquid–liquid extraction (LLX) involving tributyl phosphate (TBP) and trioctyl amine (TOA) was used for the separation of NCA 192,193Hg and NCA 192,193Au radionuclides. Walther et al. [14] separated 197mHg produced from 18 MeV proton irradiated Au target by solvent extraction using methylisobutyl ketone (MIBK)-HCl system in which Au was extracted by MIBK. A 40% (w/w) Aliquat-336 based TEVA resin (50–100 μm) was used as extractant to separate 197Hg produced from 15 MeV proton irradiated Au foil by anion exchange mechanism [15]. Both NCA Hg and bulk were strongly extracted at 1 M HNO3 and beyond 10 M HNO3 only 197Hg gets eluted while Au remained adsorbed on the resin. This behaviour was explained by the formation of anionic complex by Hg at low acid concentration and neutral complexes at high acid concentrations causing the pH selective adsorption of Hg whereas Au formed strongly anionic species. Walther et al. [16] separated NCA 197mHg and Au by solid phase extraction using LN resin (HDEHP impregnated onto an inert support) with a separation factor as high as 50,000. Studies exploring greener alternatives for separation of radioactive Hg from Au were also reported. Ghosh et al. explored the potential of room temperature ionic liquids (RTILs) in the separation of NCA 195m,g,197mHg from bulk Au target either by using water soluble ionic liquid-salt (1-butyl-3-methylimidazolium chloride-K3PO4/K2CO3) based systems [17] or hydrophobic ionic liquids-mineral acid (1-butyl-3-methylimidazoliumhexafluorophosphate or 1-butyl-3-methylimidazoliumbis(trifluoromethylsulfonyl)imide-HNO3/HCl) systems [12]. Lahiri and Roy [10] used aqueous biphasic systems i.e. polyethylene glycol (PEG) with five different sodium salts, to separate bulk Au, 197mHg from NCA 197,199−201Pb and 197,199−201Tl radionuclides produced via 197Au(7Li,xn) reactions. Subsequently, 197mHg was separated from bulk Au, by shaking the PEG rich phase containing both Au and Hg with 2 M H2SO4 and zinc metal to selectively precipitate bulk Au over Hg. Other greener approaches using the biopolymer chitosan and cloud point extraction techniques were studied to establish that chitosan and mixed micelle (Triton-X-114 and cetyltrimethylammonium bromide) are efficient reagents for adsorption of 195m,g,197mHg [18].
On the other hand, recently, we have used the resins TK200 and DGA-N to separate bulk Bi and Pb from proton irradiated lead bismuth eutectic (LBE) target [19]. Both the resins were effective in extracting Bi selectively from nitric acid medium and separating Pb and Bi. The success of TK200 and DGA-N resin in separation of Pb-Bi pair inspired us to extend the study to Hg/Au systems. In this work, 195,195m,197mHg radioisotopes were produced by 197Au(p,xn) reaction and attempt has been made to separate the bulk Au from NCA Hg radioisotopes by the use of TK200 and DGA-N. The DGA-N is a resin comprised of inert polymer beads loaded with N,N,N,N-tetra-n-octyldiglycolamide or TODGA whereas TK200 resin is based on trioctylphosphine oxide (TOPO). Structures of these two resins are available in our earlier paper [19]. Sato and Nakamura [20] studied the stability constant of the chloro- complex of Hg(II) along with Zn(II), Cd(II) by LLX with TOPO. This study was further expanded by Sato et al. [21] in which the extraction of stable Hg(II) by the same extractant was studied both from HCl and HNO3 media. These studies involved the extraction of only Hg by the reagents. To the best of our knowledge, this is the first report on the solid phase extraction using TOPO and TODGA based resins to separate NCA Hg radioisotopes from bulk Au target.
Experimental
Reagents
Au foil (99.95% purity, 0.025 mm thick) was procured from Alfa Aesar and its thickness was reduced to 5 mg/cm2 by rolling at TIFR target laboratory, Mumbai. Commercially available solid phase extraction resins DGA Normal or DGA-N (100–150 μm) and TK200 (100–150 μm), were procured from TrisKem International. All other reagents were of analytical grade.
Irradiation and data acquisition
The Au foil was irradiated with 20 MeV proton beam for 10.25 h with an integrated charge of 3929 μC at Bhabha Atomic Research Centre-Tata Institute of Fundamental Research (BARC-TIFR), Pelletron facility, Mumbai, India. The maximum production cross sections of 195Hg, 195mHg and 197mHg radioisotopes on proton induced reactions on gold target were found at higher energies i.e. 27.5 MeV, 27.1 MeV and 12.3 MeV, respectively [22, 23], but due to limitation of the terminal voltage of the pelletron, we irradiated at the maximum available energy. The energy degradation of the incident projectile energy was calculated by SRIM [24]. The exit energy of the proton beam was 19.94 MeV. Beam current was measured by Faraday cup placed at the rear of the target in combination with a current integrator. Post irradiation, the irradiated target was kept aside for 1 h to avoid instant exposure to high dose. Before dissolution of the sample, series of gamma spectra were taken in a p-type HPGe portable detector of 2.62 keV resolution at 1.33 MeV in combination with a digital spectrum analyzer (DSA 1000, CANBERRA) and Genie 2 K software (CANBERRA). The radionuclides were identified from their corresponding photo peak and decay data. The energy and efficiency calibrations of the detector were performed using 152Eu (T1/2 = 13.53 a) source of known strength.
Radiochemical separation
After taking series of spectra, the proton irradiated Au foil was dissolved in 4 mL aqua regia, evaporated to dryness and the residue was dissolved in 2 mL of 1 M HNO3 to prepare the radioactive stock solution. Solid–Liquid extraction studies were carried out in batch mode using TK200 and DGA-N in which different sets of 0.1 g dry DGA-N and TK200 were separately conditioned in 1.9 mL HNO3 for 30 min prior to extraction studies. In each set, 0.1 mL of radioactive stock was added to 1.9 mL HNO3 of different concentration followed by shaking at 40 rpm for 10 min and settling for 10 min. From the supernatant solution, 0.5 mL were taken out carefully by using syringe filters and counted in HPGe detector for 500 s each. The extent of extraction was measured by comparing with standard solution which was prepared in the same way but without shaking with the resins.
Results and discussions
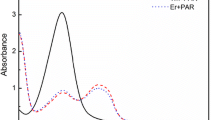
The gamma spectrum revealed the production of 195,195m,197mHg along with 196Au in the target matrix (Fig. 1). The photopeak of 197Hg at 77.35 keV was also visible. However, this peak is also populated with the X-rays of 195Hg 195mHg and 197mHg, therefore was not considered for quantitative analysis. The nuclear characteristics of each of the produced radioisotopes have been mentioned in Table 2. The yields of the produced radionuclides at the end of the bombardment (EOB) have been given in Table 3.
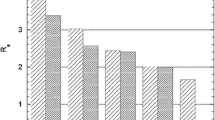
The Kd values \(\left( {{\mathbf{K}}_{{\text{d}}} = \frac{{{\text{Initial counts }} - {\text{Counts after adsorption}}}}{{\text{Counts after adsorption}}} \times \frac{{{\text{Volume of solution }}\left( {{\text{mL}}} \right)}}{{{\text{Mass of adsorbent }}\left( {\text{g}} \right)}}} \right)\) of bulk Au and NCA Hg with TK200 and DGA-N over a range of HNO3 concentration, i.e. 0.05–3 M have been plotted in Fig. 2a, b. The figures reveal that both the resins extract Au (III) quantitatively over the entire range of HNO3 concentration. The TK200 resin extracted Hg quantitatively at lower HNO3 concentration but with increase in HNO3 concentration, the extraction of Hg decreased and no extraction of NCA Hg radionuclides was observed at 3 M HNO3 concentration. The results are in accordance with Sato et al. [21] who revealed that with increase in HNO3 concentration the distribution coefficient of Hg(II) decreases when using TOPO as extractant. They concluded that the principal species of Hg(II) extracted from higher HNO3 concentration by TOPO is H2Hg(NO3)4·2TOPO. The following equilibrium equations may be responsible for extraction of Hg(II) and Au(III) from HNO3 solution by TOPO:
In the present case, TOPO based resin, TK200, might be selective towards [Au(NO3)4]− ion over [Hg(NO3)4]2− in higher acid concentration (i.e., at 3 M HNO3 concentration), which resulted in very high separation between bulk Au and NCA Hg radionuclides. The TK200 resin contains P =O bond, which makes it an excellent complexing agent.
On the other hand, the DGA-N resin also extracted Au quantitatively over the entire range of HNO3 concentration studied. However, for Hg, the extent of extraction was ~ 95% till 1 M HNO3 concentration and sharply decreased to 40% at 3 M HNO3 concentration. The oxygen donor hard bases present in diglycolamides (DGA-N) bind the cations in high oxidation state by Hard-Soft-Acid–Base (HSAB) principle. Complex formation takes place between Au (III) and DGA-N through the three oxygen donor groups. Under the experimental conditions, separation between bulk Au and NCA Hg radionuclides could not be achieved with DGA-N. The distribution coefficients of Hg and Au have been presented at the best separation condition in Table 4.
Conclusion
This is the first report on the extraction and separation of NCA Hg radioisotopes from bulk Au by the two solid phase extractants TK200 and DGA-N from nitric acid solutions. Comparative analysis of the extraction efficiency of the two resins revealed that both are suitable for carrying out extractions of either Hg or Au but TK200 resin serves as an excellent separating agent for NCA Hg radionuclides from bulk Au target. This method offers the advantage of availability of NCA Hg radioisotopes in the aqueous phase after radiochemical separation, thereby reducing secondary steps such as back extraction. The process reported in this paper does not involve any organic solvent, therefore can be considered as a clean process. The radiochemical method developed for separation of NCA Hg radionuclide is also fast and single step.
References
Elmaghraby EK, Hassan KF, Omara H, Saleh ZA (2010) Production of the mercury-197 through proton induced reaction on gold. Appl Radiat Isotopes 68:1694–1698
Neves M, Kling A, Oliveira A (2005) Radionuclides used for therapy and suggestion for new candidates. J Radioanal Nucl Chem 266:377–384
Tárkányi F, Ditrói F, Hermanne A, Takács S, Király B, Yamazaki H, Baba M, Mohammadi A, Ignatyuk AV (2011) Activation cross-sections of deuteron induced nuclear reactions on gold up to 40 MeV. Nucl Instrum Meth Phys Res B 269:1389–1400
Tárkányi F, Hermanne A, Ditrói F, Takács S, Adam Rebeles R, Ignatyuk AV (2015) New data on cross-sections of deuteron induced nuclear reactions on gold up to 50 MeV and comparison of production routes of medically relevant Au and Hg radioisotopes. Nucl Instrum Meth Phys Res B 362:116–132
Tilbury RS, Yaffe L (1963) Nuclear isomers Hg197m, g, Hg195m, gand Au196m, g formed by bombardment of gold with protons of energies from 8 to 60 MeV. Can J Chem 41:2634–2642
Ditrόi F, Tárkányi F, Takács S, Hermanne A (2016) Activation cross sections of proton induced nuclear reactions on gold up to 65 MeV. Appl Radiat Isotopes 113:96–106
Szelecsѐnyi F, Steyn GF, Kovács Z, Van der Walt TN (2007) Application of Au + p nuclear reactions for proton beam monitoring up to 70MeV. Intl Conf Nucl Data Sci Technol. https://doi.org/10.1051/ndata:07379
Hermanne A, Tárkányi F, TakácsS SYN, Kovalev S (2006) Experimental determination of activation cross section of alpha-induced nuclear reactions on natPt. Nucl Instrum Meth Phys Res B 251:333–334
Sudar S, Qaim SM (2006) Cross sections for the formation of 195Hgm, g, 197Hgm, g, and 196Aum, g in α and 3He-particle induced reactions on Pt: Effect of level density parameters on the calculated isomeric cross-section ratio. Phys Rev C 73:034613
Lahiri S, Roy K (2009) A green approach for sequential extraction of heavy metals from Li irradiated Au target. J Radioanal Nucl Chem 281:531–534
Nayak D, Lahiri S, Ramaswami A (2002) Alternative radiochemical heavy ion activation methods for the production and separation of thallium radionuclides. Appl Radiat Isotopes 57:483–489
Ghosh K, Maiti M, Lahiri S (2017) Separation of 195(m, g),197mHg from bulk gold target by liquid-liquid extraction using hydrophobic ionic liquids. RadiochimActa 105:747–753
Lahiri S, Banerjee K, Das NR (1999) Production of carrier free 192,193Hg and 192,193Au in 16O irradiated tantalum target and their separation by liquid-liquid extraction. J Radioanal Nucl Chem 242:497–504
Walther M, Preusche S, Bartel S, Wunderlich G, Freudenberg R, Steinbach J, Pietzsch HJ (2015) Theranostic mercury: 197(m)Hg with high specific activity for imaging and therapy. Appl Radiat Isotopes 97:177–181
Despotopulos JD, Kmak KN, Gharibyan N, Brown TA, Grant PM, Henderson RA, Moody KJ, Tumey SJ, Shaughnessy DA, Sudowe R (2016) Production and isolation of homologs of flerovium and element 115 at the Lawrence Livermore National Laboratory Center for Accelerator Mass Spectrometry. J Radioanal Nucl Chem 308:567–572
Walther M, Lebeda O, Preusche S, Pietzsch HJ, Steinbach J (2017) Theranostic mercury part 1: a new Hg/Au separation by a resin based method. AIP Conf Proc 1845:020023
Ghosh K, Lahiri S, Maiti M (2016) Separation of no-carrier-added 195(m, g),197mHg from Au target by ionic liquid and salt based aqueous biphasic systems. J Radioanal Nucl Chem 310:1345–1351
Mandal S, Nayak D (2010) Production, separation and speciation of no-carrier-added Hg radionuclides using greener methodologies. RadiochimActa 98:45–51
Choudhury D, Lahiri S, Nag T, Sodaye S, Bombard A (2020) Separation of bulk Pb and Bi from proton irradiated lead bismuth eutectic (LBE) target by DGA-N and TK200 resins. J Radioanal Nucl Chem 324:897–902
Sato T, Nakamura T (1980) The stability constants of the aqueous chloro complexes of divalent zinc, cadmium and mercury determined by solvent extraction with tri-n-octylphosphine oxide. Hydrometallurgy 6:3–12
Sato T, Sato K, Noguchi Y (2001) Liquid-Liquid extraction of mercury (II) from hydrochloric acid and nitric acid solutions by trioctylphosphine oxide. Shigen-to-Sozai 117:880–884
Nagame Y, Sueki K, Baba S, Nakahara H (1990) Isomeric yield ratios in proton-, 3He-, and α-particle-induced reactions on 197Au. Phys Rev C 41:889
Satheesh B, Musthafa MM, Singh BP, Prasad R (2012) Study of isomeric cross-section ratio and pre-equilibrium fraction in proton and alpha particle induced nuclear reactions on 197Au. Int J Mod Phys E 21:1250059
Zeigler JF, Bierserk JP (2013) The stopping and range of ions in matter (SRIM). Pergammon Press, New York
Acknowledgements
Authors are thankful to BARC-TIFR Pelletron staffs and TIFR target laboratory staffs for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choudhury, D., Lahiri, S., Nag, T.N. et al. Separation of no-carrier-added 195,195m,197mHg from proton irradiated Au target by TK200 and DGA-N resins. J Radioanal Nucl Chem 327, 1299–1303 (2021). https://doi.org/10.1007/s10967-021-07610-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07610-5