Abstract
In this study, extraction and separation of thorium(IV) and a few representative rare earths in HNO3 media was evaluated using trioctylmethylammonium nitrate ([A336][NO3]) ionic liquid. An unexpected novel extraction mechanism was identified based on the studies in the slope analysis and ESI–MS spectrum. The trimer of [A336][NO3] was confirmed to dominate the extraction reaction by ESI–MS spectrum before and after the extraction. The extraction efficiency of La(III), Eu(III) and Lu(III) by [A336][NO3] are much lower than Th(IV) in acid medium, which means that it is possible to separate thorium from rare earths or the rare earth ores by using [A336][NO3].
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Along with growing environmental concern and the increasing demand of energy from fossil fuel, nuclear energy is considered as an attractive option for solving the global energy crisis in the future because of its high energy density and low greenhouse gas emission [1, 2]. Thorium is a potential nuclear fuel because 232Th can convert to 233U by absorbing slow neutrons [3]. It has being paid more and more attention due to the ongoing consumption of uranium resources [4]. Generally, the rare earth ores such as monazite, bastnasite, and xenotime are natural deposits of lanthanides containing thorium. To eliminate the radioactive pollution caused by thorium and the widespread use of rare earth, it is always necessary for the extraction and separation of thorium and rare earths [5]. As we known, solvent extraction is a major technique for the separation of actinides and rare earths in the industrial field, which has been widely used in hydrometallurgy [6, 7], waste disposal [8, 9], and environmental treatment [10]. The solvent extraction can be used to decontaminate the rare earth concentrates from thorium-bearing radioactive material or to produce nuclear fuel [11]. In these processes, various extractants such as amines [12, 13], organophosphorus compounds [14, 15] have been developed for the extraction and separation of thorium and rare earths. There are reasons to believe that the production of new thorium-based nuclear fuel and the reprocessing of thorium-based spent fuel using the solvent extraction would become an important issue in the future.
Room temperature ionic liquids (RTILs or ILs) are a class of organic salts composed of ions that can also be liquid at or near room temperature. Due to some useful solvent properties, including their high thermal stability, negligible volatility, high selectivity, and high solvent extraction efficiency, RTILs are increasingly recognized and accepted as a kind of novel green solvents for liquid–liquid extraction [16,17,18,19]. RTILs have shown some appealing applications in the nuclear fuel cycle especially as an alternative to molecular diluent in solvent extraction procedures [20,21,22]. Most of the ILs typically used for solvent extraction processes contain imidazolium, pyridinium, ammonium, and phosphonium cations, because these ILs tend to be immiscible with water in combination with some anions. Some studies demonstrated that long-chain quaternary ammonium and phosphonium ionic liquids are thermal and chemical stability compared to their imidazolium- and pyridinium-based counterparts [23]. Aliquat® 336-based ILs, trioctylmethylammonium chloride([A336][Cl]) are highly hydrophobic due to their long-chain alkyl groups. Thus, Aliquat-336 is regarded as a versatile and cheap cation source for the synthesis of a new family of hydrophobic ionic liquids. The cation present in Aliquat® 336, trioctylmethylammonium cation ([A336]+) can be combined with several anions by simple replacement of the chloride ions [24]. Therefore, some of these ionic liquids have already been studied as diluent or extractant in solvent extraction and the mechanisms of extraction have been also reported [20, 25,26,27].
The previous studies revealed that either cations or anions of ILs may act as more interesting roles in an extraction process, respectively [28]. Due to the fact that Aliquat® 336-based ILs ([A336][Cl]) more commonly exist as semi-solid or solid at room temperature, we wish to investigate the extraction and separation of thorium and rare earths using the corresponding nitrate ionic liquid [A336][NO3] (trioctylmethylammonium nitrate) derived from [A336][Cl]). In fact, the pure [A336][NO3]) is a viscous liquid at room temperature [23], which is more suitable for the extraction of metal ions (Fig. 1). Thus, the effects of several experimental parameters, including the aqueous acidity, diluent and extractant concentration, were evaluated in terms of the ligands and the metal ions in this study. The new extraction mechanism was discussed using a slope analysis method, and were further confirmed by ESI–MS spectrum. Also, the stripping of the thorium ions from the loaded organic phase was investigated.
Experimental
Chemicals and apparatus
All chemicals and reagents were of analytical grade and used without further purification. All work solutions were obtained by appropriate dilution of the standardized stock solutions. Aliquot 336 (methyltrioctyl/decylammonium chloride, > 97% quaternary ammonium content) was supplied by Aladdin (China) and also not purified. Standard solutions of metal ions were prepared by dissolving corresponding oxides (99.99%) with concentrated nitric acid and diluting with ultra-pure water. Th(NO3)4·5H2O (from Aladdin, China) were used as the sources of Th(IV) for the following extraction.
The metals were analyzed using an inductively coupled plasma optical emission spectrometer (ICP-OES, IRIS Advantage ER/S, TJA, USA). High-resolution mass spectra (HRMS) were recorded on a Bruker micrOTOF-QII analysis instrument.
Synthesis of [A336][NO3]
[A336][NO3] was synthesized according to the modified literature methods [23]. 100 g of [A336][Cl] (trioctylmethylammonium chloride) was mixed with 100 mL dichloromethane (DCM) to form a solution of organic phase, which was pre-equilibrated four times with 200 mL of 2.5 M KNO3 solution each time in order to exchange the chloride ions to nitrate ions. During these four ion-exchange steps, the organic phase was always separated by separating funnel from the mixture each time. After the fourth equilibration, chloride levels were minimal and almost unchanged. It indicated that the majority of chloride ions in [A336][Cl] had been converted to nitrate ions. After the metathesis reaction, the resulting ionic liquid was washed further with 100 mL of water to remove chloride and KNO3 impurities. Finally, a yellow and viscous [A336][NO3] ionic liquid was obtained by evaporating DCM under reduced pressure (yield: 95%).
Extraction procedure
For solvent extraction, HNO3 solution containing metal ions and [A336][NO3] solution (in various diluents) were taken in a 1:1 volume ratio and equilibrated on a mechanical shaker for the required time. The solution was allowed to settle for 20 min. The preliminary experiments have confirmed that 20 min shaking is enough to complete the equilibrium at 25 ± 1 °C. After centrifugation (5 min and 2000 rpm/min) and separation of both phases, the duplicate aliquots were taken from the aqueous phases. The amount of metal ions in aqueous solutions was determined by ICP-OES. The distribution ratio (D) is the ratio of the concentration of metal ions in the organic phase to the concentration of metal ions in the aqueous phase after solvent extraction. Thorium (or other metal ions) concentration in the organic phase was, in turn, calculated from the difference of initial concentration of thorium taken in aqueous phase and the thorium left in the aqueous phase after solvent extraction. The data in this study were an average of triplicate measurements in mass balance within ± 5%. The distribution ratio (D) of metal ions was calculated based on the following Eq. (1):
The extraction efficiency (%E) was determined by using Eq. (2):
where [Mn+]org represents the concentrations of metal ions in the loaded organic phase and [Mn+]aq represents the concentrations of metal ions in the aqueous phase after the equilibrium.
The separation factor (SF) was calculated as Eq. (3):
where DTh(IV) represents the distribution ratio of Th(IV) ions and DLn(III) represents the distribution ration of La(III), Eu(III) and Lu(III) ions, respectively.
Results and discussion
Effect of contacting time
The extraction kinetics of Th(IV) was investigated by 5.04 × 10−4 M in 4.0 M HNO3 solution contacting with 0.05 M [A336][NO3] in xylene. It is distinct from Fig. 2 that the extraction efficiency of Th(IV) increases quickly with increasing of contacting time, and almost reached equilibrium after 10 min. Further extending time to 20 min, no obvious change in %E is observed. The result indicated that 20 min is sufficient up to equilibrium for the extraction process. Thus, 20 min of equilibration time of two phases have been employed for all the following extraction process to ensure the complete extraction.
Effect of diluents and HNO3 concentration
The appropriate solvent plays an important role in performing a satisfactory extraction process. The expected properties of extraction solvents are high distribution ratio, good solubility to extractant and low solubility in water. Initially, the extraction behavior of Th(IV) ions in several organic solvents, such as chloroform (CHCl3), 1,2-dichloralethane (CH2ClCH2Cl), xylene and dichloromethane (CH2Cl2) were studied as a function of the HNO3 concentration (1.0–6.0 M) in the aqueous phase. Apolar and weak polar solvents are preferred because of quick separation between the diluent and the aqueous phase. The concentration of HNO3 had a significant impact on the extraction efficiency of thorium(IV), and the distribution ratios of DTh(IV) increased with the increase of nitric acid concentration for all the diluents used. As shown in Fig. 3, the extraction efficiency for all diluents reached the highest levels when 6.0 M HNO3 was employed as the result of aqueous solution. It can be also observed that xylene had the highest extraction efficiency compared to the other solvents used. Besides, the extraction efficiency of thorium(IV) by pure [A336][NO3] was also investigated, and an outstanding DTh(IV) values higher than 2000 was obtained under high acidity. However, it is distinct that the high viscosity of [A336][NO3] hinders the extraction process due to the poor mass transfer. The complete equilibrium was extended to an increasingly longer 60 min in pure [A336][NO3] system. Therefore, because of the high compatibility, low volatility, excellent solubility and shorter equilibrium time as well as excellent extractability, the weak polar xylene was selected for the subsequent experiments.
Effect of extractant content and HNO3 concentration
More experiments were performed for a comprehensive understanding of the extraction process of thorium(IV) ion. Figure 4 represents the relation between HNO3 concentration and distribution ratio of Th(IV) by using 0.10, 0.15, 0.20, 0.30, 0.40 and 0.50 M [A336][NO3] in xylene from aqueous HNO3 solutions containing Th(IV) ions. In order to investigate the effect of [A336][NO3] concentration on extraction efficiency, several experiments were carried out with the HNO3 concentration ranged from 1.0 to 6.0 mol/L. It was clear that the extraction efficiency for all diluents reached the highest levels when 6.0 M HNO3 was employed as the result of aqueous solution. The distribution ratio of Th(IV) using 6.0 M nitric acid increased from 68.79 to 1105 with the increase of [A336][NO3] concentration from 0.10 to 0.50 M. Similar to the distribution ratio, the extraction efficiency of Th(IV) increases from 98.57% to almost 100% after 20 min equilibrium. It was worth noting that, using 0.5 M [A336][NO3], the distribution ratio of Th(IV) reached almost 91.83 even in 1 M nitric acid medium. In addition, when the ionic liquid concentration increased from 0.2 to 0.5 M, the distribution ratio showed a rapid increase. We hypothesized that [A336][NO3] ionic liquids are more likely to form trimers at high concentrations, thus resulting in higher extraction efficiency. All the above results indicate that the content of [A336][NO3] in the organic phase has a positive effect on the Th(IV) extraction efficiency. Due to the excellent performance in the present system, the extraction mechanisms of [A336][NO3] in xylene for Th(IV) were further investigated.
Extraction mechanism
Slope analysis is the most commomly used method for the determination of the number of extractant molecules involved in the extraction process. A slope analysis was performed by extracting 5.04 × 10−4 M Th(IV) ions in aqueous phase using [A336][NO3] as extractant. In the meantime, xylene was chosen as organic phase but the total volume of the organic phase was kept constant. Figure 5 showed the variation in the distribution ratio of Th(IV) as a function of ionic liquid concentration in organic phase. It is observed that the DTh increased with the increase in the concentration of ionic liquid. Linear regression analysis of the extraction data resulted in a slope of ca. 2 at different acidity, which is in agreement with the result reported [1]. The previous mechanism for extraction of thorium by Aliquat 336 was written as Eq. (4):
To establish the extraction equilibrium of the system, the main extractable species should be considered. Therefore, the determining ESI–MS of organic phase was performed before or after extraction of Th(IV) with [A336][NO3] in the present study. The data were acquired on a Bruker microOTOF-QII system. In positive ion mode of mass spectrometry (Fig. 6), peak at m/z 798.8356 can be observed using organic phase after the extraction, which is corresponding to the representative extracted species ([(A336)2(NO3)]+, calcd. m/z: 798.8391). Similarly, peak at m/z 541.9790 (Fig. 7) could be assigned to the corresponding negative ions ([(Th(NO3)5]−, calcd. m/z: 542.0626). Using the organic phase before extraction, peaks at m/z 798.8365 (Fig. 8) as [(A336)2(NO3)]+ were also observed in positive ion mode. Peak at m/z 492.3919 (Fig. 9) could be explicitly assigned to the corresponding negative ions ([(A336)(NO3)2]−, calcd. m/z: 492.4013). The above results suggest that the first trimerization of [A336][NO3] partially forms the trimeric species ([(A336)2(NO3)]+[(A336)(NO3)2]−) to participate into the extraction reaction. Although Nasab [1] presumed that the thorium complex was extracted with two molecules of Aliquat 336, and the extractable complex of Th(NO3) 2−6 involved in the extraction reaction. Clearly, no peak was corresponding to Th(NO3) 2−6 in our study by the ESI mass spectrum. It indicates that the ion-pairing interactions between various charged organic or inorganic species are more complex than those represented in Eq. (3). According to the above results, the extraction equilibrium for thorium can be represented by the following updated reactions rather than the reported Eq. (4):
Extraction of Th(IV) from a mixture solution
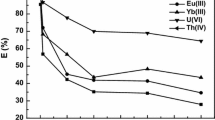
To test the selectivity of the present extractant, a competitive extraction of Th(IV) was investigated from a mixture solution containing thorium(IV), lanthanide(III), europium(III) and lutetium(III) using [A336][NO3] in xylene. As showed in Fig. 10, the present extraction system exhibited a high affinity to tetravalent thorium ion at high acidity. When HNO3 concentration reached up to 6.0 mol/L, the distribution ratios of Th(IV) was as high as 1000. In the meantime, the extraction of La(III), Eu(III) and Lu(III) is negligible and nearly invariable with HNO3 concentration ranging from 1.0 M to 6.0 M. It is clear from Fig. 10 that the selective extractions were accomplished in the order: Th(IV) ≫ La(III) ≈ Eu(III) ≈ Lu(III). As listed in Table 1, the good to excellent separation factor (SF) between Th(IV) and typical rare earths was obtained in the [A336][NO3]-xylene system. The highest separation factor (SF) values of Th(IV)/La(III), Th(IV)/Eu(III) and Th(IV)/Lu(III) were reached in 8.78 × 103, 9.01 × 103 and 8.80 × 103, respectively. Therefore, it is possible to separate Th(IV) from rare earths by the [A336][NO3] ionic liquid.
Stripping properties
After the extraction at 6.0 M HNO3, the organic phase (initial concentration of [A336][NO3] was 0.50 M) loaded with Th(IV) was back-extracted using different reagents such as diluted HNO3, Na2SO4, NaNO3 as well as pure water. As showed in Table 2, the loaded thorium could be easily stripped using the above reagents. The stripping efficiency for 0.1 M HNO3, 1.0 M Na2SO4, 1.0 M NaNO3, pure water was 94.5, 99.6, 95.4, 94.4 at one stage, respectively. It can be attributed to not easily form [(Th(NO3)5]− complex anions at low acidity. Among these, 1.0 M Na2SO4 seems to be the most effective stripping reagent, and almost a complete stripping (100%) of Th(IV) can be achieved by twice contacts.
Conclusions
The present study proposed a facile liquid–liquid extraction method for separation of thorium and rare earth elements. The optimum conditions for thorium separations from lanthanum, europium and lutetium were investigated using [A336][NO3] in nitric acid solution. The distribution ratios (D) of Th(IV) increased with increasing the concentration of HNO3. [A336][NO3] exhibited a high selectivity and extraction power for Th(IV) over Lanthanides(III) in high HNO3 concentration. The slope analysis method was conducted to investigate the ion-association extraction mechanisms. A novel extraction mechanisms were unexpectedly observed. The trimer of [A336][NO3] was confirmed to involve into the extraction reaction by ESI–MS spectrum before and after the extraction. Complete stripping and regeneration of the ionic liquid could be performed by using several chemicals or pure water by a single or twice contact. The extraction efficiency of La(III), Lu(III) and Eu(III) by [A336][NO3] are much lower than Th(IV) at high acidity, which means that the present system can be developed to separate thorium from lanthanum, lutetium or europium.
References
Nasab ME (2014) Solvent extraction separation of uranium(VI) and thorium(IV) with neutral organophosphorus and amine ligands. Fuel 116:595–600
Rao A, Tomar BS (2016) Extraction of thorium employing N,N-dialkyl amide into room temperature ionic liquid followed by supercritical carbon dioxide stripping. Sep Purif Technol 161:159–164
Ashley SF, Parks GT, Nuttall WJ, Boxall C, Grimes RW (2012) Thorium fuel has risks. Nature 492:31–33
Li Z, Zhao H, He SH, Chen M, Li QA (2016) Systematic research on solvent extraction process for extracting 233U from irradiated thorium. Hydrometallurgy 166:160–166
Nasab ME, Sam A, Milani SA (2011) Determination of optimum process conditions for the separation of thorium and rare earth elements by solvent extraction. Hydrometallurgy 106:141–147
Kuang S, Zhang Z, Li Y, Wu G, Wei H, Liao W (2017) Selective extraction and separation of Ce(IV) from thorium and trivalent rare earths in sulfate medium by an α-aminophosphonate extractant. Hydrometallurgy 167:107–114
Yan Z-Y, Huang Q-G, Wang L, Zhang F (2019) Synthesis of tailored bis-succinamides and comparison of their extractability for U(VI), Th(IV) and Eu(III). Sep Purif Technol 213:322–327
Raju CSK, Subramanian MS (2007) Sequential separation of lanthanides, thorium and uranium using novel solid phase extraction method from high acidic nuclear wastes. J Hazard Mater 145:315–322
McCann K, Mincher BJ, Schmitt NC, Braley JC (2017) Hexavalent actinide extraction using N,N-dialkyl amides. Ind Eng Chem Res 56:6515–6519
Ren P, Wang C, Tao W, Yang X, Yang S, Yuan L, Chai Z, Shi W (2020) Selective separation and coordination of europium(III) and americium(III) by bisdiglycolamide ligands: solvent extraction, spectroscopy, and DFT calculations. Inorg Chem 59:14218–14228
Ibrahim SM, Zhang Y, Xue Y, Yang S, Ma F, Tian G (2020) Extraction of lanthanides(III) along with thorium(IV) from chloride solutions by N,N-di(2-Ethylhexyl)-diglycolamic acid. Solvent Extr Ion Exc 38:417–429
Rout A, Venkatesan KA, Srinivasan TG, Rao PRV (2012) Ionic liquid extractants in molecular diluents: extraction behavior of europium(III) in quarternary ammonium-based ionic liquids. Sep Purif Technol 95:26–31
Zuo Y, Chen J, Li DQ (2008) Reversed micellar solubilization extraction and separation of thorium(IV) from rare earth(III) by primary amine N1923 in ionic liquid. Sep Purif Technol 63:684–690
Gupta B, Malik P, Deep A (2002) Extraction of uranium, thorium and lanthanides using Cyanex-923: their separations and recovery from monazite. J Radioanal Nucl Chem 251:451–456
Nanda D, Oak MS, Maiti B, Chauhan HP, Dutta PK (2002) Selective and uphill transport of uranyl ion in the presence of some base metals and thorium across bulk liquid membrane by di (2-ethylhexyl) phosphoric acid. Sep Sci Technol 37:3357–3367
Niu Y-N, Ren P, Zhang F, Yan Z-Y (2018) Solvent extraction of Eu3+ with 4-oxaheptanediamide into ionic liquid system. Sep Sci Technol 53:2750–2755
Errico M, Eduardo SR, Juan JQR, Rong BG, Juan GSH (2017) Multi objective optimal acetone-butanol-ethanol (ABE) separation systems using liquid–liquid extraction assisted divided wall columns. Ind Eng Chem Res 56:11575–11583
Yan Z-Y, Ren P, Huang Q-G, He M-J, Li Y, Wu Z-M, Wu W-S (2016) Solvent extraction of uranyl ion with 4-oxaheptanediamide into ionic liquid system from HNO3 solution. J Radioanal Nucl Chem 310:703–709
Dai S, Luo J, Li J, Zhu X, Cao Y, Komarneni S (2017) Liquid-liquid micro-extraction of Cu2+ from water using a new circle micro-channel device. Ind Eng Chem Res 56:12717–12725
Shkrob IA, Marin TW, Jensen MP (2014) Ionic liquid based separations of trivalent lanthanide and actinide. Ind Eng Chem Res 53:3641–3653
Depuydt D, Dehaen W, Binnemans K (2015) Solvent extraction of scandium(III) by an aqueous biphasic system with a nonfluorinated functionalized ionic liquid. Ind Eng Chem Res 54:8988–8996
Sun X, Luo H, Dai S (2011) Ionic liquids-based extraction: a promising strategy for the advanced nuclear fuel cycle. Chem Rev 112:2100–2128
Zhu M, Zhao J, Li Y, Mehio N, Qi Y, Liu H, Dai S (2015) An ionic liquid-based synergistic extraction strategy for rare earths. Green Chem 17:2981–2993
Leyma R, Platzer S, Jirsa F, Kandioller W, Krachler R, Keppler BK (2016) Novel thiosalicylate-based ionic liquids for heavy metal extractions. J Hazard Mater 314:164–171
Hoogerstraete TV, Binnemans K (2014) Highly efficient separation of rare earths from nickel and cobalt by solvent extraction with the ionic liquid trihexyl(tetradecyl)phosphonium nitrate: process relevant to the recycling of rare earths from permanent magnets and nickel metal hydride batteries. Green Chem 16:1594–1606
Hoogerstraete TV, Wellens S, Verachtert K, Binnemans K (2013) Removal of transition metals from rare earths by solvent extraction with an undiluted phosphonium ionic liquid: separations relevant to rare-earth magnet recycling. Green Chem 15:919–927
De los Ríos AP, Hernández-Fernández FJ, Alguacil FJ, Lozano LJ, Ginestá A, García-Díaz I, Sánchez-Segado S, López FA, Godínez C (2012) On the use of imidazolium and ammonium-based ionic liquids as green solvents for the selective recovery of Zn(II), Cd(II), Cu(II) and Fe(III) from hydrochloride aqueous solutions. Sep Purif Technol 97:150–157
Sun T, Xu C, Fu J, Chen Q, Chen J, Shen X (2017) Extraction of U(VI) by the ionic liquid hexyltributylphosphonium bis (trifluoromethylsulfonyl)imides: an experimental and theoretical study. Sep Purif Technol 188:386–393
Acknowledgements
The authors thank the National Natural Science Foundation of China (Grant No. 21471072) for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, X., Zhang, F., Wu, Q. et al. The separation of thorium and rare earth elements using [A336][NO3]: insight into a new extraction mechanism. J Radioanal Nucl Chem 327, 1251–1258 (2021). https://doi.org/10.1007/s10967-020-07590-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07590-y