Abstract
Common agro-waste, potato peels were used to study the efficacy of adsorption of long-lived radioisotopes, 133Ba (T1/2= 10.54 a) and 134Cs (T1/2 = 2.06 a) as a precursor of fission products 140Ba (T1/2 = 12.75 d) and 137Cs (T1/2 = 30.07 a). At optimized condition of pH = 3, high Kd value > 3 × 104 cm3 g−1 was observed for Ba, when 60 mg dried potato peel was used as sorbent-material for adsorption from a binary aqueous solution containing 133Ba and 134Cs. Cs showed low Kd values across the entire pH range. The bio-sorbent could effectively uptake ~ 99% of 133Ba and ~ 48% of 134Cs at the best condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental protection from hazardous materials is always a concern and challenge. Heavy metal pollution remains one of the most explored fields of research since aquatic systems get easily contaminated with industrial-domestic run-offs or effluents thereby distressing the floral-faunal communities. Conventional techniques like ion exchange, reverse osmosis, membrane filtration, etc., have broad application in this field [1, 2]. Besides, environmentally benign and eco-friendly methods like phytoremediation [3,4,5], use of natural bio-sorbents, bio-degradable materials [6,7,8,9,10], etc., are also providing efficient, safe, economical way of bio-remediation.
Similar to heavy metals, radioactive waste materials pose a serious threat to the environment and its components if not properly stored or managed. Many industrial, medical, research and nuclear sectors regularly use radioactive materials for various purposes. Even at trace concentrations, radioisotopes may exhibit chemical and radiological toxicity to living organisms [11]. In this regards, radioactive waste management is both demanding and challenging. Radio-caesium is one of the major long-lived elements in liquid radioactive wastes (LRW). Apart from generated wastes, nuclear fall-outs, activities or nuclear accidents contribute significantly towards radioisotopic contamination of the surrounding, which is also definitely a cause for concern [12, 13]. Plenty of research and modeling related to extent of radioactive contamination has been reported following nuclear accidents like Chernobyl, Fukushima-Daichii, etc. [14,15,16,17,18,19]. In most of the reports, emphasis has been put on scrutinizing the contamination, migration and residence properties of labile caesium (134Cs, 137Cs), which is easily transportable [14, 20,21,22,23]. Cs being labile and soluble gets easily transferred upward along the food chain, eventually entering the human system. Hence, several biological, chemical and physical methods have been undertaken to study adsorption or extraction behavior of such long-lived isotopes [24, 25].
Long-lived radionuclides like 133Ba (T1/2 = 10.54 a), 134Cs (T1/2 = 2.06 a) and fission products like 137Cs (T1/2 = 30 a) and 140Ba (T1/2 = 12.75 d), when released in the environment, may lead to radioactive contamination in the surrounding. In fact, both 137Cs and 140Ba have high fission yield (Table 1). It is always imperative to develop efficient techniques towards separation or absorption of such radioisotopes. Hence, studies on extraction behavior of caesium and barium radioisotopes are of interest and importance.
In fact, cheaper and accessible substitutes like chitosan, alginates, agricultural wastes, wood wastes, clay materials, etc., are becoming useful sorbent-materials. Calcium alginate beads had been studied for uptake of 133Ba from a binary mixture of 133Ba and 134Cs [27]. Radioactive Ba and Cs have been removed by several bio-adsorbents efficiently including mosses, seeds, agricultural wastes, plant materials, bio-materials (beads, chitosan), etc. [28,29,30,31,32,33,34].
The aim of present study is primarily to investigate the efficacy of a low-cost, easily available potato-peel (Solanum tuberosum) wastes for adsorption of the most labile fission products 137Cs. However, the fission yield of the adjacent 140Ba is equal to that of 137Cs. Therefore, adsorption probability of 140Ba on potato peel was also considered. We have used binary mixture of 133Ba and 134Cs, which would act as precursor of 140Ba and 137Cs respectively. In this experiment, 137Cs has been purposefully avoided as it is in secular equilibrium with 137Ba (T1/2 = 2.52 min), which shares the same \(\gamma\) line with 137Cs at 661.6 keV and would confuse the adsorption pattern between Ba and Cs radionuclides. The experimental parameters like pH, time of contact, amount of adsorbent, etc., affecting the adsorption behavior have been optimized.
Materials and methods
Chemicals and reagents
All the chemicals used for the present study were of analytical grade. De-ionized water (18.2 MΩ cm) was obtained from ThermoScientific smart2 Pure 6 UV/UF water purification system. Emparta concentrated HNO3, procured from Merck, India was used for the experiment.
Potatoes were purchased from local market and washed thoroughly. Afterwards the peels were separated from potato and were again thoroughly flushed under running tap water for removal of unwanted dirt. Next, these were rinsed in de-ionized water and thereafter soaked in sufficient volume of 0.1 M HNO3 for surface protonation. Cleansed peels were left overnight, completely air-dried, also oven-dried and finally pulverized into fine powder, sieved (mesh no. 100) and used as the bio-sorbent for 133Ba and 134Cs adsorption.
133Ba and 134Cs radionuclides were procured from Board of Radiation and Isotope Technology (BRIT), Mumbai. A binary stock solution of 5 mL was prepared by mixing 133Ba and 134Cs. From this stock, every time 100 µL of activity was taken for each batch of extraction process.
Instruments
p-type, coaxial well-type high-purity germanium (HPGe) detector (make CANBERRA) having 30% relative efficiency and 2.7 keV resolution at 1332 keV energy was used for all the radiochemical measurements. The detector is placed inside 5 cm thick lead cylindrical rings with 0.2 cm steel shield inside. The associated electronics, DSA-1000 was procured from CANBERRA. Energy calibration of the system was performed using point sources of 152Eu and 60Co. Efficiency calibration was done inside the well with a liquid 152Eu source of known strength to maintain the same geometry of the samples. Gamma-spectra obtained were analyzed using Genie 2000 software. For the batch experiment, 1.5 mL of sample was taken in Eppendorf and counted inside the well, and stripped off the respective backgrounds. 133Ba was radiometrically monitored from its γ-peak at 81 keV, whereas 604.7 keV γ peaks were considered for 134Cs.
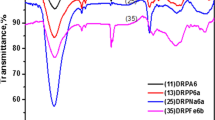
Functional groups present in the bio-sorbent (PP) was determined by FTIR spectroscopy. IR spectra of dried potato peels in KBr were recorded using a Perkin-Elmer FTIR RX1 spectrometer (4500−500 cm− 1).
Radiochemical extraction
A binary stock solution of 5 mL was prepared by mixing 133Ba and 134Cs. From this stock, every time 100 µL of activity was taken for each batch of extraction process. All measurements were done inside the well of the HPGe detector. This allowed us to work with minimum amount of radioactivity. The activity of 134Cs and 133Ba in each 100 µL solution were 64 and 200 Bq, respectively.
Adsorptions of 133Ba and 134Cs were studied in batch mode by powdered potato peel (PP). Solid liquid extraction (SLX) was performed with 3.9 mL HNO3 solution of different concentrations, (0.0001, 0.001, 0.01, 0.1 and 1 M) as aqueous phase and 20 mg PP as solid phase. 0.1 mL binary stock solution containing 133Ba and 134Cs was added to experimental samples, mechanically shaken for 10 min followed by 10 min centrifugation at 5000 rpm. After phase separation, 1.5 mL supernatant was taken in Eppendorf for gamma-spectroscopic study.
Shaking time (5, 10, 20, 30, 40, 60 min) was varied keeping the amount of activity (0.1 mL), amount of adsorbent (20 mg PP) and acidity of the solution (10− 3 M HNO3) fixed. Similarly, weight variation of the adsorbent was done by varying the amount of PP (5, 20, 40, 60, 80, 100 mg) keeping shaking time (10 min) and acidity of the solution (10− 3 M HNO3) fixed. After centrifugation, 1.5 mL of supernatant was taken out. For every set of experiment, the extent of adsorption by PP was calculated by comparing against reference solution.
Results and discussions
Dried potato peel showed adsorption of both 133Ba and 134Cs from the binary solution. Adsorption trend is similar for both the radioisotopes, but adsorption of 133Ba by PP is higher compared to that of Cs. The adsorption profile of 133Ba and 134Cs at different acidic concentrations has been shown in Fig. 1. Maximum adsorption of barium (~ 92%) was observed at 10− 3 M HNO3 acid concentration followed by steady decrease in adsorption reaching minimum at 0.1 M concentration, followed by slight increase at 1 M HNO3 concentration. A steady decrease on adsorption of 134Cs was observed with increasing acid concentration.
Adsorption of 133Ba and 134Cs at different shaking times did not show much variation. Initially adsorption had increased for both 133Ba and 134Cs but after 10 min, adsorption process was almost saturated.
Keeping the HNO3 concentration fixed at 10− 3 M and shaking and settling time 10 min, the amount of bio-adsorbent was varied (Fig. 2). Initially the extent of adsorption increased with increasing amount of adsorbent and reached a saturation after 60 mg adsorbent. When 60 mg PP was taken, the extent of adsorption for 133Ba was ~ 99.8% and that of 134Cs was ~ 48%. With further increase in the amount of sorbent-material (80, 100 mg), uptake of 134Cs increased to ~ 50%. Adsorption (%) and distribution coefficients, Kd (cm3 g− 1) of 133Ba and 134Cs radionuclides by the sorbent-material at different conditions have been provided in Table 2. The distribution coefficient Kd of the radioisotopes by the sorbent-material has been calculated from the following equation:
Similar to Fig. 2, Kd values decreased for both Ba and Cs radionuclides with increase in acid concentration. With variation in sorbent weight from 5 to 100 mg, V/m ratio varied from 800 to 40 cm3/g. The maximum Kd value for Ba (3 × 104) was obtained when 60 mg PP was used as sorbent at pH 3 (10 min shaking and settling time). The Kd value of Cs remained low (~ 102) in the entire experiment which indicates a good separation between Ba and Cs radionuclides might be possible.
In Table 3, the present adsorption data has been compared with few reported bio-sorbents used for removal of Ba and Cs from aqueous solutions [32, 35,36,37,38,39,40,41,42,43,44,45,46,47]. Kd values obtained for Ba adsorption by dried potato peel is better when compared to Ba adsorption by Aloe vera waste. At best condition, Kd values for Ba for Aloe vera waste, acid treated Aloe vera waste and base treated Aloe vera waste were 360, 380 and 1310 cm3 g− 1 respectively [32]. Also, PP is efficient in terms of Kd values when compared to that of calcium alginate (CA) beads. At pH = 7, 10 min shaking time, 60 CA beads, Kd value of of Ba was found to be 86.48 cm3 g− 1 [27].
The extent of Cs sorption in the present experiment is comparable to that of mucilaginous seed biomass, which was reported to extract 40% of Cs at pH = 6.5 [43]. Examples of some other reported bio-sorbents are edible mushroom (Pleurotus citrinopileatus species), which was found to adsorb 137Cs [48]; common yeast, Saccharomyces cerevisiae, which could selectively uptake long-lived 152,154Eu from a mixture of 152,154Eu, 60Co and 134Cs without accumulation of 60Co and 134Cs [49]; marine cyanobacteria for Cs(I) sorption as reported by Yu et al. [50], etc.
The absorption mechanism onto bio-sorbents is a complex process and multiple mechanism like electrostatic interaction, ion exchange or adduct formation with the surface functional groups may work together. For the present experiment, a careful observation to the Fig. 1 may vouch against the electrostatic attraction. A point of zero charge (PZC) is observed in case of electrostatic attraction. It is the point beyond which no metal-adsorbent interaction occurs. From Fig. 1, it is clear that there is no PZC for Cs. Though at 0.1 M concentration Ba adsorption is zero, but beyond 0.1 M adsorption starts again, which should not be the case for electrostatic attraction.
The adsorption process observed in the present experiment can be explained by the ion exchange mechanism, where both Ba2+ and Cs+ are in competition for the binding sites. pH is one of the significant factors affecting the adsorption process. Due to the higher charge density of Ba2+, it easily replaces H+ from protonated PP surface as compared to Cs+ and adsorption of 133Ba is more than that of 134Cs under all experimental conditions. According to Tran et al. [51], bio-sorbents exhibit a low specific surface area and total pore volume but have abundant surface functional groups, and a high net surface charge density. We have analysed dried PP under FTIR spectroscopy. Analysis of the sorbent-material showed a strong peak at 3332 cm− 1, which confirms the presence of H-bonded –OH stretching. The hydroxyl group might belong to carboxylic acid, phenol or alcohols, which are present within lignin, hemicellulose and cellulose moieties of the adsorbent. –C=O stretching of aldehydes and ketones was observed at 1591 cm− 1. Potato peels generally consist of lignocellulosic residues comprising of cellulose, hemicellulose and lignin. These components possess different functional groups such as phenolic, hydroxyl, carboxylic, amines and ether groups, which can also contribute to physical and chemical interactions via surface complexation, adduct formation or hydrogen bonding [52, 53]. The adsorbent is rich with oxygenated surface groups, which can easily attract and bind cations like Ba2+ or Cs+. Bivalent Ba2+ naturally is more efficient for these interactions.
Conclusions
Some thrown away agro-wastes have already been established as adsorbent for long-lived radionuclides. Earlier, we have studied the efficacy of potato peel towards adsorption of 88Zr radioisotope [54, 55]. The present experiment successfully added potato peel (Solanum tuberosum) to the list as adsorbent of 134,137Cs and 133,140Ba radionuclides. At the optimum conditions of pH = 3, 60 mg adsorbent, 10 min shaking time and 298 K, maximum > 99% of 133Ba was adsorbed by dried potato peel. Kd (cm3 g− 1) value calculated at this point was 30,388 cm3 g− 1. Under the same condition, maximum 48% of 134Cs was adsorbed by dried potato peel. However, poor Kd (cm3 g− 1) values were obtained for 134Cs. It is true that the maximum adsorption for 134Cs was ~ 50% in the present experimental condition. But multiple extraction by potato peel would increase the extent of extraction. For example, three times extraction would lead to about 87% extraction of caesium radionuclides. Hence, this study is an attempt to investigate the efficacy of potato peel as a bio-adsorbent for Ba and Cs radionuclides. However, bio-sorbents, by definition are bio-degradable and is not compatible with inorganic matrices with respect to cementation and classing processes for final deposition of radionuclides.
References
Qdais HA, Moussa H (2004) Removal of heavy metals from wastewater by membrane processes: a comparative study. Desalination 164:105–110
Lakherwal D (2014) Adsorption of heavy metals: a review. Int J Environ Res Dev 4:41–48
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Mukherjee G, Saha C, Naskar N, Mukherjee A, Mukherjee A, Lahiri S et al (2018) An endophytic bacterial consortium modulates multiple strategies to improve arsenic phytoremediation efficacy in Solanum nigrum. Sci Rep 8:1–16
Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I et al (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nat Biotechnol 13:468–474
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14
Vakili M, Rafatullah M, Salamatinia B, Abdullah AZ, Ibrahim MH, Tan KB et al (2014) Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review. Carbohydr Polym 113:115–130
Maity S, Naskar N, Lahiri S, Ganguly J (2019) Polysaccharide–derived hydrogel water filter for the rapid and selective removal of arsenic. Environ Sci: Water Res Technol. DOI:https://doi.org/10.1039/C9EW00247B
Ghosh K, Naskar N, Choudhury D, Lahiri S (2019) Natural flavonoids as superior reagents for separation of clinically important Zr radionuclides. ChemRxiv
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40
Chen Y, Wang J (2012) The characteristics and mechanism of Co(II) removal from aqueous solution by a novel xanthate-modified magnetic chitosan. Nucl Eng Design 242:452–457
Steinhauser G, Brandl A, Johnson TE (2014) Comparison of the Chernobyl and Fukushima nuclear accidents: a review of the environmental impacts. Sci Total Environ 470:800–817
Kryshev II (1995) Radioactive contamination of aquatic ecosystems following the Chernobyl accident. J Environ Radioact 27:207–219
Vakulovsky SM, Nikitin AI, Chumichev VB, Katrich IY, Voitsekhovich OA, Medinets VI et al (1994) Cesium-137 and strontium-90 contamination of water bodies in the areas affected by releases from the Chernobyl nuclear power plant accident: an overview. J Environ Radioact 23:103–122
Hou XL, Fogh CL, Kucera J, Andersson KG, Dahlgaard H, Nielsen SP (2003) Iodine-129 and Caesium-137 in Chernobyl contaminated soil and their chemical fractionation. Sci Total Environ 308:97–109
Kinoshita N, Sueki K, Sasa K, Kitagawa JI, Ikarashi S, Nishimura T et al (2011) Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc Natl Acad Sci 108:19526–19529
Manolopoulou M, Vagena E, Stoulos S, Ioannidou A, Papastefanou C (2011) Radioiodine and radiocesium in Thessaloniki, Northern Greece due to the Fukushima nuclear accident. J Environ Radioact 102:796–797
Bolsunovsky A, Dementyev D (2011) Evidence of the radioactive fallout in the center of Asia (Russia) following the Fukushima nuclear accident. J Environ Radioact 102:1062–1064
Christoudias T, Lelieveld J (2013) Modelling the global atmospheric transport and deposition of radionuclides from the Fukushima Daiichi nuclear accident. Atmos Chem Phys 13:1425
Masumoto Y, Miyazawa Y, Tsumune D, Tsubono T, Kobayashi T, Kawamura H et al (2012) Oceanic dispersion simulations of 137Cs released from the Fukushima Daiichi nuclear power plant. Elements 8:207–212
Takahashi J, Tamura K, Suda T, Matsumura R, Onda Y (2015) Vertical distribution and temporal changes of 137Cs in soil profiles under various land uses after the Fukushima Dai-ichi Nuclear Power Plant accident. J Environ Radioact 139:351–361
Ohta T, Mahara Y, Kubota T, Fukutani S, Fujiwara K, Takamiya K et al (2012) Prediction of groundwater contamination with 137Cs and 131I from the Fukushima nuclear accident in the Kanto district. J Environ Radioact 111:38–41
Inoue M, Kofuji H, Hamajima Y, Nagao S, Yoshida K, Yamamoto M (2012) 134Cs and 137Cs activities in coastal seawater along Northern Sanriku and Tsugaru Strait, northeastern Japan, after Fukushima Daiichi Nuclear Power Plant accident. J Environ Radioact 111:116–119
Kaygun A, Eral M, Akyil S (2017) Removal of cesium and strontium using natural attapulgite: evaluation of adsorption isotherm and thermodynamic data. J Radioanal Nucl Chem 311:1459–1464
Attallah MF, Allan KF, Mahmoud MR (2016) Synthesis of poly(acrylic acid maleic acid) SiO2/Al2O3 as novel composite material for cesium removal from acidic solutions. J Radioanal Nucl Chem 307:1231–1241
Nuclear data safeguards, Cumulative fission yields, https://www.IAEA.org
Mandal A, Lahiri S (2011) Separation of 134Cs and 133Ba radionuclides by calcium alginate beads. J Radioanal Nucl Chem 290:115–118
Krishna MVB, Rao SV, Arunachalam J, Murali MS, Kumar S, Manchanda VK (2004) Removal of 137Cs and 90Sr from actual lowlevel radioactive waste solution using moss as a phyto-sorbent. Sep Purifi Technol 38:149
Mishra SP, Prasad SK, Dubey RS, Mishra M, Tiwari D, Lee SM (2007) Biosorptive behaviour of rice hulls for Cs-134 from aqueous solutions: A radiotracer study. Appl Radiat Isot 65:280–286
Omar HA, Attia LAEB (2013) Kinetic and equilibrium studies of cesium-137 adsorption on olive waste from aqueous solutions. Radiochemistry 55:497–450
Mishra S, Tiwari D, Prasad S, Dubey R, Mishra M (2007) Biosorptive behavior of mango (Mangifera indica) and neem (Azadirachta indica) barks for 134Cs from aqueous solutions: A radiotracer study. J Radioanal Nucl Chem 272:371–379
Kapashi E, Kapnisti M, Dafnomili A, Noli F (2019) AloeVera as an effective biosorbent for the removal of thorium and barium from aqueous solutions. J Radioanal Nucl Chem 321:217–226
Sarkar K, Sen K, Lahiri S (2017) Separation of long-lived 152Eu radioisotopes from a binary mixture of 152Eu and 134Cs by calcium alginate: a green technique. J Radioanal Nucl Chem 311:2001–2006
Maity S, Datta A, Lahiri S, Ganguly J (2015) Selective separation of 152Eu from a mixture of 152Eu and 137Cs using a chitosan based hydrogel. RSC Advances 5:89338–89345
Younis SA, El-Salamony RA, Tsang YF, Kim KH (2020) Use of rice straw-based biochar for batch sorption of barium/strontium from saline water: Protection against scale formation in petroleum/desalination industries. J Clean Prod 250:119442
Majidnia Z, Idris A, Majid M, Zin R, Ponraj M (2015) Efficiency of barium removal from radioactive waste water using the combination of maghemite and titania nanoparticles in PVA and alginate beads. Appl Radiat Isot 105:105–113
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J Hazard Mat 190:916–921
Noli F, Kapnisti M, Buema G, Harja M (2016) Retention of barium and europium radionuclides from aqueous solutions on ash-based sorbents by application of radiochemical techniques. Appl Radiat Isot 116:102–109
Khandaker S, Toyohara Y, Kamida S, Kuba T (2018) Adsorptive removal of cesium from aqueous solution using oxidized bamboo charcoal. Water Resour Ind 19:35–46
El-Din AM, Monir T, Sayed MA (2019) Nano-sized Prussian blue immobilized costless agro-industrial waste for the removal of cesium-137 ions. Environ Sci Pollut Res 26:25550–25563
Gurung M, Adhikari BB, Alam S, Kawakita H, Ohto K, Inoue K, Harada H (2013) Adsorptive removal of Cs(I) from aqueous solution using polyphenols enriched biomass-based adsorbents. Chem Eng J 231:113–120
Attia LA, Youssef MA, Abdel Moamen OA (2019) Feasibility of radioactive cesium and europium sorption using valorized punica granatum peel: kinetic and equilibrium aspects. Sep Sci Technol 28:1–6
Sangurdekar PR, D’Souza SF, Melo JS (2001) Biosorption of radioactive cobalt and caesium using mucilaginous seeds. In: Proceedings of the tenth national symposium on environment
Ivanets AI, Shashkova IL, Drozdova NV, Davydov DY, Radkevich AV (2014) Recovery of cesium ions from aqueous solutions with composite sorbents based on tripolite and copper (II) and nickel (II) ferrocyanides. Radiochemistry 56:524–528
Maslova M, Mudruk N, Ivanets A et al (2020) A novel sorbent based on Ti-Ca-Mg phosphates: synthesis, characterization, and sorption properties. Environ Sci Pollut Res 27:3933–3949
Oleksiienko O, Levchuk I, Sitarz M, Meleshevych S, Strelko V, Sillanpää M (2016) Adsorption of caesium (Cs+) from aqueous solution by porous titanosilicate xerogels. Desalin Water Treat 57:5554–5566
Ali MS, Sami NM, El-Sayed AA (2020) Removal of Cs+, Sr2+ and Co2+ by activated charcoal modified with Prussian blue nanoparticle (PBNP) from aqueous media: kinetics and equilibrium studies. J Radioanal Nucl Chem. doi.https://doi.org/10.1007/s10967-020-07067-y
Mukhopadhyay B, Nag M, Laskar S, Lahiri S (2007) Accumulation of radio-cesium by Pleurotus citrinopileatus species of edible mushroom. J Radioanal Nucl Chem 272:415–418
Roy K, Sinha P, Lahiri S (2008) Immobilization of long-lived radionuclides 152,154Eu by selective bioaccumulation in Saccharomyces cerevisiae from a synthetic mixture of 152,154Eu, 137Cs and 60Co. Biochem Eng J 40:363–367
Yu R, Chai H, Yu Z, Wu X, Liu Y, Shen L et al (2020) Behavior and mechanism of Cesium Biosorption from aqueous solution by living Synechococcus PCC7002. Microorganisms 8:491
Tran HN, Nguyen HC, Woo SH, Nguyen TV, Vigneswaran S, Hosseini-Bandegharaei A et al (2019) Removal of various contaminants from water by renewable lignocellulose-derived biosorbents: a comprehensive and critical review. Crit Rev Env Sci Technol 49:2155–2219
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY et al (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour Technol 148:574–583
Asuquo ED, Martin AD (2016) Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: Characterisation, kinetic and isotherm studies. J Env Chem Eng 4:4207–4228
Naskar N, Choudhury D, Basu S, Banerjee K (2019) Separation of NCA 88Zr from proton irradiated natY target: a novel approach using low cost bio–sorbent potato peel charcoal. J Radioanal Nucl Chem DOI. https://doi.org/10.1007/s10967-019-06637-z
Naskar N, Basu S, Banerjee K (2019) Analysis of isotherms and thermodynamic parameters for Zr(IV) adsorption from aqueous medium using potato peel charcoal (PPC). In: 15th International symposium on metal ions and organic pollutants in biology, medicine and environment, NEERI, Nagpur, India, Oct 30–31
Acknowledgements
Authors are grateful to Professor Susanta Lahiri, Head, Chemical Sciences Division, Saha Institute of Nuclear Physics (SINP) for scientific discussion and extending the facilities. Authors are thankful to Dr. Rajib Sarkar, Department of Chemistry, Prabhu Jagatbandhu College, Howrah, India, for the FTIR analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naskar, N., Banerjee, K. Development of sustainable extraction method for long-lived radioisotopes, 133Ba and 134Cs using a potential bio-sorbent. J Radioanal Nucl Chem 325, 587–593 (2020). https://doi.org/10.1007/s10967-020-07241-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07241-2